Summary
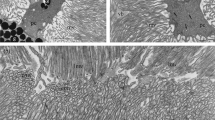
Hemicholinium-3 (HC-3), a drug which prevents synthesis of acetylcholine in neurons, when injected intraperitoneally in doses as low as 2×5 mg/kg produces marked ultrastructural changes and damage in rod but not in cone photoreceptors. In rods it causes a reduction in cytoplasmic back-ground density, swelling of the endoplasmic reticulum, ballooning of the outer membrane of the nucleus, leaching of the nucleoplasm and clumping of the nuclear chromatin. In dark-adapted rods HC-3 produces some loss of cytoplasmic synaptic vesicles but no reduction in numbers of those vesicles which lie adjacent to the synaptic ribbons. In light-adapted rods the drug does not cause such an apparent reduction of synaptic vesicles but does induce a considerable reduction in numbers of vesicles associated with the ribbons. These structural changes are discussed in the light of what is known about the pharmacology of HC-3 and neurotransmitter release from vertebrate photoreceptors.
Similar content being viewed by others
References
Ansell, G.B., Spanner, S.: The metabolism of choline in regions of rat brain and the effect of hemicholinium-3. Biochem. Pharmacol. 24, 1719–1723 (1975)
Cervetto, L., MacNichol, E.F.: Pharmacology of horizontal cells in the isolated perfused skate retina. Biol. Bull. 141, 381 (1971)
Csillik, B., Joó, F.: Effect of hemicholinium on the numbers of synaptic vesicles. Nature (Lond.) 213, 508–509 (1967)
Dacheux, R.F., Miller, R.F.: Photoreceptor-bipolar cell transmission in the perfused retina eyecup of the mudpuppy. Science 191, 963–964 (1976)
Diamond, I., Milfay, D.: Uptake of 3H-Methyl choline by microsomal, synaptosomal, mitochondrial and synaptic vesicle fractions of rat brain. The effects of hemicholinium. J. Neurochem. 19, 1899–1909 (1972)
Dickson, D.H., Flumerfelt, B.A. Hollenberg, M.J., Gwyn, D.G.: Ultrastructural localization of cholinesterase activity in the outer plexiform layer of the newt retina. Brain Res. 35, 299–303 (1971)
Dowling, J.E.: Synaptic arrangements in the vertebrate retina: the photoreceptor synapse. In: Synaptic transmission and neuronal interaction (M.V.L. Bennet, ed.), pp. 87–103. Amsterdam: North-Holland Publishing Company 1974
Dowling, J.E., Ripps, H.: Aspartate isolation of receptor potentials in the skate retina. Biol. Bull. 141, 384 (1971)
Dowling, J.E., Ripps, H.: Effect of magnesium on horizontal cell activity in the skate retina. Nature (Lond.) 242, 101–103 (1973)
Elmquist, D., Quastel, D.M.: Presynaptic action of hemicholinium at the neuromuscular junction. J. Physiol. (Lond.) 177, 463–482 (1965)
Graham, L.T.: Comparative aspects of neurotransmitters in the retina. In: The eye comparative physiology, Vol. 6 (H. Davson and L.T. Graham, eds.), pp. 283–342. New York-London: Academic Press 1974
Gray, E.G., Pease, H.L.: On understanding the organisation of the retinal photoreceptor synapses. Brain Res. 35, 1–15 (1971)
Hebb, C.O., Ling, G.M., McGeer, E.G., McGeer, P.L., Perkins, D.: Effect of locally applied hemicholinium on the acetylcholine content of the caudate nucleus. Nature (Lond.) 204, 1309–1311 (1964)
Jones, D.G., Synapses and synaptosomes morphological aspects. London: Chapman and Hall 1975
Jones, S.F., Kwanbunbumpen, S.: The effects of nerve stimulation and hemicholinium on synaptic vesicles at the mammalian neuromuscular junction. J. Physiol. (Lond.) 207, 31–50 (1970)
Kennedy, A.J., Voaden, M.: Free amino acids in the photoreceptor cells of the frog retina. J. Neurochem. 23, 1093–1095 (1974)
Lam, D.M.K.: Biosynthesis of acetylcholine in turtle photoreceptors. Proc. nat. Acad. Sci. (Wash.) 69, 1987–1991 (1972)
McIntosh, F.C.: Synthesis and storage of acetylcholine in nervous tissue. Canad. J. Biochem. Physiol. 41, 2555–2571 (1963)
Mathur, R.L., Klethi, J., Ledig, M., Mandel, P.: Cysteine sulphate carboxylase in the visual pathway of adult chicken. Life Sci. 18, 75–80 (1976)
Mollenhauer, H.H.: Plastic embedding mixtures for use in electron microscopy. Stain Technol. 39, 111–114 (1964)
Monaghan, P., Osborne, M.P.: Light induced formation of dense-core vesicles in rod photoreceptors in retina of Xenopus laevis. Nature (Lond.) 257, 586–587 (1975)
Murakami, M., Ohtsu, K., Ohtsuka, T.: Effects of chemicals on receptors and horizontal cells in the retina. J. Physiol. (Lond.) 226, 899–913 (1972)
Noell, W.K.: Aspects of experimental and hereditary retinal degeneration. In: Biochemistry of the retina (C.N. Graymore, ed.), pp. 51–72. New York: Academic Press 1965
Ohman, S., Shelley, W.B.: Induction of a unique fluorescence in the photoreceptor of the retina. Nature (Lond.) 220, 378–379 (1968)
Orr, H.T., Cohen, A.I., Lowry, O.H.: The distribution of taurina in the vertebrate retina. J. Neurochem. 26, 609–611 (1976)
Osborne, M.P., Monaghan, P.: Effects of light and dark upon the photoreceptor synapses in the retina of Xenopus laevis. Cell Tiss. Res., in the press (1976)
Párducz, A., Fehér, O., Joó, F.: Effects of stimulation and hemicholinium (HC-3) on the fine structure of nerve endings in the superior cervical ganglion of the cat. Brain Res. 34, 61–72 (1971)
Reale, E., Luciano, L., Spitznas, M.: The fine structural localization of acetylcholinesterase activity in the retina and optic nerve of rabbit. J. Histochem. Cytochem. 19, 85–96 (1971)
Rodriguez De Lores Arnaiz, G., Zieher, L.M., De Robertis, E.: Neurochemical and structural studies on the mechanism of action of hemicholinium-3 in central cholinergic synapses. J. Neurochem. 17, 221–229 (1970)
Ross, C.D., McDougal, D.B.: The distribution of choline acetyltransferase activity in vertebrate retina. J. Neurochem. 26, 521–526 (1976)
Schueler, F.W.: The mechanism of action of the hemicholiniums. Int. Rev. Neurobiol. 2, 77–97 (1960)
Stell, W.K.: The morphological organization of the vertebrate retina. In: Handbook of sensory physiology, Vol. 7 (M.C.F. Fuortes, ed.), pp. 111–214. Berlin-Heidelberg-New York: Springer 1972
Tebécis, A.K.: Transmitters and identified neurons in the mammalian central nervous system. Bristol: Scientechnica Ltd. 1974
Trifonov, Yu. A.: Study of synaptic transmission between photoreceptors and horizontal cells by means of electrical stimulation of the retina. Biofizika 13, 809–817 (1968)
Val'Tsev, V.B.: Role of cholinergic structures in outer plexiform layer in the electrical activity of frog retina. Fed. Proc. 25 (Transl. Suppl.), T765-T766 (1966)
Waloga, G., Pak, W.L.: Horizontal cell potentials: dependence on external sodium ion concentration. Science 191, 864–866 (1976)
Author information
Authors and Affiliations
Additional information
The authors wish to thank SRC for a grant in support of this work
Rights and permissions
About this article
Cite this article
Osborne, M.P., Monaghan, P. Differential sensitivity of rods and cones in Xenopus retina to hemicholinium-3. Cell Tissue Res. 175, 59–72 (1976). https://doi.org/10.1007/BF00220823
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00220823