Summary
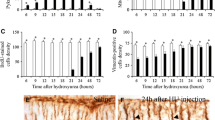
The present report describes the genesis, development and topographical distribution of ectopic cells of the external granular layer in the subarachnoid space covering the rat cerebellum. Following one intracisternal injection to newborn rats of 100 μg 6-hydroxydopamine (6-OHDA), the meningeal cells degenerate and are removed by phagocytosis within 24 h post injection (p.i.), leaving the cerebellar cortex without a pia-arachnoid cover. Defects appear in the basal lamina investing the cerebellar cortex 3 to 5 days p.i., and both external granule cells and ‘sprouts’ from Bergmann-glia endfeet grow into the subarachnoid space. The latter form large, flat glial lamellae and cover extensive areas of the denuded cerebellar surface, although they do not form a glial scar over the exposed neuropil of the cerebellar cortex. The numbers of ectopic external granule cells increase within the subarachnoid space both by proliferation and a continuous efflux of cells from the cerebellar cortex. They migrate, aggregate, and ultimately develop into granule, stellate and basket cells, the morphology of which is indistinguishable from their counterparts in situ; they make specific afferent and efferent connections, both among themselves and with the underlying cerebellar cortex and brainstem. The distribution of ectopic external granule cells and their derivatives is restricted to the anterior vermal fissures and the vermal-hemispheric junctions. The present results indicate that external granule cells and their derivatives are capable of both differentiating normally and surviving in the subarachnoid space if they become associated with glial cells and establish synaptic connections.
Similar content being viewed by others
References
Alien C, Sievers J, Berry M, Jenner S (1981) Experimental studies on cerebellar foliation. II. A morphometric analysis of cerebellar fissuration defects and growth retardation after neonatal treatment with 6-OHDA in the rat. J Comp Neurol 203:771–784
Altman J, Anderson WJ (1969) Early effects of X-irradiation of the cerebellum in infant rats. Decimation and reconstitution of the external granular layer. Exp Neurol 24:196–216
Bernfield M, Banerjee SD (1982) The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev Biol 90:291–305
Berry M, Sievers J, Baumgarten HG (1980a) The influence of afferent fibres on the development of the cerebellum. In: Di Benedetta C (ed) Multidisciplinary approach to brain development Elsevier, North-Holland Amsterdam, pp 91–106
Berry M, Sievers J, Baumgarten HG (1980b) Adaptation of the cerebellum to deafferentation. Prog Brain Res 53:65–92
Bignami A, Eng LF, Dahl D, Uyeda CT (1972) Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res 43:429–435
Björklund A, Stenevi U (1979) Regeneration of monoaminergic and cholinergic neurons in the mammalian central nervous system. Physiol Rev 59:62–99
Brun A (1965) The subpial granular layer of the foetal cerebral cortex in man. Acta Pathol Microbiol Scand (Suppl) 179:1–98
Caviness VS Jr, Rakic P (1978) Mechanisms of cortical development. A view from mutations in mice. Ann Rev Neurosci 1:297–326
Caviness VS Jr, Evrard P, Lyon G (1978) Radial neuronal assemblies, ectopia and necrosis of developing cortex. A case analysis. Acta Neuropathol 41:67–72
Cerro M del, Walker JR, Stoughton RL, Cosgrove JW (1976) Displaced neural elements within the cerebellar fissures of normal and experimental albino rat. Anat Rec 184:389
Chan-Palay V (1972) Arrested granule cells and their synapses with mossy fibers in the molecular layer of the cerebellar cortex. Z Anat Entwickl-Gesch 139:11–20
Cohen AL, Marlow DP, Garner GE (1968) A rapid critical point method using fluorocarbons (freons) as intermediate and transitional fluids. J Microsc 7:331–342
Ebels EJ (1970) The influence of age upon the effect of early postnatal X-irradiation on the development of the cerebellar cortex in rats. Acta Neuropathol 15:298–307
Ebels EJ (1972) Studies on ectopic granule cells in the cerebellar cortex — with a hypothesis as to their aetiology and pathogenesis. Acta Neuropathol 21:117–127
Evrard P, Caviness VS Jr, Prats-Vinas J, Lyon G (1978) The mechanism of arrest of neuronal migration in the Zellweger malformation. An hypothesis based upon cytoarchitectonic analysis. Acta Neuropathol 41:109–117
Fischer J, Gutmann E (1949) A case of heterotopia of undifferentiated nervous tissue by the way of subarachnoidal implantation. Cs Rentgenel 4:171–180
Freeman W (1926) Cortical heterotopia in the pontine meninges. Arch Pathol Lab Med 2:352–354
Friede RL (1975) Gross and microscopic development of the central nervous system. In: Developmental neuropathology, Springer, Wien New York, pp 1–23
Galaburda AM, Kemper TL (1978) Cytoarchitectural abnormalities in developmental dyslexia: a case study. Ann Neurol 6:94–100
Griffin WST, Eriksson MAE, del Cerro M, Woodward DJ, Stampfer N (1980) Naturally occurring alterations of cortical layers surrounding the fissura prima of rat cerebellum. J Comp Neurol 192:109–118
Hámori J (1969) Development of synaptic organization in partially agranular and in transneuronally atrophied cerebellar cortex. In: Llinas R (ed) Neurobiology of cerebellar evolution and development. Am Med Ass, Chicago, pp 845–858
Hicks SP, D'Amato CJ (1961) How to design and build abnormal brains using radiation during development. In: Fields WS, Desmond MM (eds) Disorders of the developing nervous system Thomas, Springfield, pp 60–93
Ito S, Winchester RJ (1963) The fine structure of gastric mucosa in the bat. J Cell Biol 16:541–578
Jellinger K (1972) Embryonal cell nests in human cerebellar nuclei. Z Anat Entwickl Gesch 138:145–154
Jones KL, Smith DW (1975) The fetal alcohol syndrome. Teratology 12:1–10
Jonsson G (1980) Chemical neurotoxins as denervation tools in neurobiology. Ann Rev Neurosci 3:169–187
Kilham L, Margolis G (1966) Viral etiology of spontaneous ataxia of cats. Am J Pathol 48:991–1006
Landis SC (1973) Granule cell heterotopia in normal and nervous mutant mice of the balb/c strain. Brain Res 61:175–189
Larsell O (1952) The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J Comp Neurol 97:231–256
Lidov HGW, Molliver ME (1982) The structure of cerebral cortex in the rat following prenatal administration of 6-hydroxydopamine. Dev Brain Res 3:81–108
Lipton JM (1966) Locomotor behaviour and neuromorphologic anomalies in prenatally and postnatally irradiated rats. Radial Res 28:822–828
Luft JH (1961) Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol 9:409–414
Mangold U, Sievers J (1981) Differentiation of cerebellar external granular layer cells in the subarachnoid space. Acta Anat 111:95
Mares V, Lodin Z (1970) The cellular kinetics of the developing mouse cerebellum. II. The function of the external granular layer in the process of gyrification. Brain Res 23:343–352
Mestres P, Rascher K (1981) Supraependymal cell clusters in the rat brain. Cell Tissue Res 218:41–58
Monjan AA, Gilden DH, Cole GA, Nathanson N (1971) Cerebellar hypoplasia in neonatal rats caused by lymphocytic choriomeningitis virus. Science 171:194–196
Nathanson N, Cole GA, van der Loos H (1969) Heterotopic cerebellar granule cells following administration of cyclophosphamide to suckling rats. Brain Res 15:532–536
Pfaffenroth MJ, Das GD (1974) Heterotopic cells nests in the developing rat cerebellum. Acta Neuropathol 30:1–9
Phemister RD, Shively JN, Young S (1969a) The effects of gamma irradiation on the postnatally developing canine cerebellar cortex. I. Effects of single sublethal exposures. J Neuropathol Exp Neurol 28:119–127
Phemister RD, Shively JN, Young S (1969b) The effects of gamma irradiation on the postnatally developing canine cerebellar cortex. II. Sequential histogenesis of radiation-induced changes. J Neuropathol Exp Neurol 28:128–138
Rakic P (1975) Cell migration and neuronal ectopias in the brain. In: Birth defects. Original Article Series, vol XI, pp 95–129
Reynolds ES (1962) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Robain O, Lyon G (1972) Les micrencéphalies familiales par malformation cérébrale. Etude anatomoclinique. Acta Neuropathol 20:96–109
Rosenstein JM, Brightman MW (1981) Anomalous migration of central nervous tissue to transplanted autonomic ganglia. J Neurocytol 10:387–409
Seymour RM, Berry M (1975) Scanning and transmission electron microscope studies of interkinetic nuclear migration in the cerebral vesicles of the rat. J Comp Neurol 160:105–126
Shimada M, Langman J (1970a) Repair of the external granular layer after postnatal treatment with 5-fluorodeoxyuridine. Am J Anat 129:247–260
Shimada M, Langman J (1970 b) Repair of the external granular layer of the hamster cerebellum after prenatal and postnatal administration of methylazoxymethanol. Teratology 3:119–134
Sievers J (1979) Experimentelle Studien zur Frage der intrazerebralen Induktion. Der Locus coeruleus und sein terminales Zielgebiet, das Kleinhirn. Verh Anat Ges 73:1159–1160
Sievers J, Mangold U (1981) Ektope cerebellare Neurone im Subarachnoidalraum — ein neues Modell für die Neurobiologie? Anat Anz 149:100
Sievers J, Baumgarten HG, Berry M, Klemm HP, Jenner S (1979) The locus coeruleus neuroblasts. Plasticity and function in development. In: Usdin E, Kopin IJ, Barchas J (eds) Catecholamines. Basic and clinical frontiers. Pergamon, New York, pp 848–850
Sievers J, Klemm HP, Jenner S, Baumgarten HG, Berry M (1980) Neuronal and extraneuronal effects of intracisternally administered 6-hydroxydopamine on the developing rat brain. J Neurochem 34:765–771
Sievers J, Berry M, Baumgarten HG (1981 a) The role of noradrenergic fibers in the control of post-natal cerebellar development. Brain Res 207:200–208
Sievers J, Mangold U, Berry M, Allen C, Schlossberger HG (1981 b) Experimental studies on cerebellar foliation. I. A qualitative morphological analysis of cerebellar fissuration defects after neonatal treatment with 6-OHDA in the rat. J Comp Neurol 203:751–769
Sievers H, Sievers J, Baumgarten HG, König N, Schlossberger HG (1983) Distribution of tritium label in the neonate rat brain following intracisternal or subcutaneous administration of 3H-6-OHDA. An autoradiographic study. Brain Research, in press
Sosa JM, Palacios E, de Sosa HMS (1971) Heterotopic cerebellar granule cells inside the plexiform layer. Acta Anat 80:91–98
Spacek J, Parizek J, Lieberman AR (1973) Golgi cells, granule cells and synaptic glomeruli in the molecular layer of the rabbit cerebellar cortex. J Neurocytol 2:407–428
Sumi SM, Hager H (1968a) Electron microscopic study of the reaction of the newborn rat brain to injury. Acta Neuropathol 10:324–335
Sumi SM, Hager H (1968b) Electron microscopic features of an experimentally produced porencephalic cyst in the rat brain. Acta Neuropathol 10:336–346
Stenevi U, Björklund A, Svendgaard NA (1976) Transplantation of central and peripheral monoamine neurons to the adult brain: techniques and conditions for survival. Brain Res 114:1–20
Stoughton RL, del Cerro M, Walker JR, Swarz JR (1978) Presence of displaced neural elements within rat cerebellar fissures. Brain Res 148:15–29
Volpe JJ, Adams RD (1972) Cerebro-hepato-renal syndrome of Zellweger. An inherited disorder of neuronal migration. Acta Neuropathol 20:175–198
Watson WE (1976) Cell biology of brain. Chapman and Hall, London, pp 1–40
Willis RA (1958) The borderland of embryology and pathology. Butterworth, London, pp 331–334
Yamamoto M, Chan-Palay V, Steinbusch HWM, Palay SL (1980) Hyperinnervation of arrested granule cells produced by the transplantation of monoamine-containing neurons into the fourth ventricle of rat. Anat Embryol 159:1–15
Yu WHA (1977) The effect of 5-bromodeoxyuridine on the postnatal development of the rat cerebellum: morphologic and radioautographic studies. Am J Anat 150:89–108
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. H. Leonhardt on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Sievers, J., Mangold, U. & Berry, M. 6-OHDA-induced ectopia of external granule cells in the subarachnoid space covering the cerebellum. Cell Tissue Res. 230, 309–336 (1983). https://doi.org/10.1007/BF00213807
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213807