Summary
This paper describes measurements of electrical potentials generated by renal Na/K-ATPase reconstituted into proteoliposomes, utilizing the anionic dye, oxonol VI. Calibration of absorption changes with imposed diffusion potentials allows estimation of absolute values of electrogenic potentials.
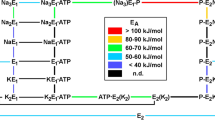
ATP-dependent Nacyt/Kexc exchange in K-loaded vesicles generates large potentials, up to 250 mV. By comparing initial rates or steady-state potentials with ATP-dependent22Na fluxes in different conditions, it is possible to infer whether coupling ratios are constant or variable. For concentrations of Nacyt (2–50mm) and ATP (1–1000 μm) and pH's (6.5–8.5), the classical 3Nacyt/2Kexc coupling ratio is maintained. However, at low Nacyt concentrations (<0.8mm), the coupling ratio is apparently less than 3Nacyt/2Kexc.
ATP-dependent Nacyt/congenerexc exchange in vesicles loaded with Rb, Cs, Li and Na is electrogenic. In this mode congeners, including Naexc, act as Kexc surrogates in an electrogenic 3Nacyt/2congenerexc exchange. (ATP+Pi)-dependent Kcyt/Kexc exchange in K-loaded vesicles is electroneutral.
ATP-dependent “uncoupled” Na flux into Na- and K-free vesicles is electroneutral at pH 6.5–7.0 but becomes progressively electrogenic as the pH is raised to 8.5. The22Na flux shows no anion specificity. We propose that “uncoupled” Na flux is an electroneutral 3Nacyt/3Hexc exchange at pH 6.5–7.0 but at higher pH's the coupling ratio changes progressively, reaching 3Na/no ions at pH 8.5. Slow passive pump-mediated net K uptake into Na- and K-free vesicles is electroneutral, and may also involve Kcyt/Hexc exchange.
We propose the general hypothesis that coupling ratios are fixed when cation transport sites are saturated, but at low concentrations of transported cations, e.g., Nacyt in Na/K exchange and Hexc in “uncoupled” Na flux, coupling ratios may change.
Similar content being viewed by others
References
Admon, A., Shahak, Y., Avron, M. 1982. Adenosine triphosphate-generated transmembrane electrical potential in chloroplasts.Biochim. Biophys. Acta 681:405–411
Apell, H.-J., Bersch, B. 1987. Oxonol VI as an optical indicator for membrane potentials in lipid vesicles.Biochim. Biophys. Acta 903:480–494
Bashford, C.L., Chance, B., Smith, J.C., Yoshida, T. 1979. The behaviour of oxonol dyes in phospholipid dispersions.Biophys. J. 25:63–85
Bashford, C.L., Smith, J.C. 1978. The use of optical probes to monitor membrane potential.Methods Enzymol. 55:569–586
Blostein, R. 1983a. The influence of cytoplasmic sodium concentration on the stoichiometry of the sodium pump.J. Biol. Chem. 258:12228–12232
Blostein, R. 1983b. Sodium pump-catalysed sodium-sodium exchange associated with ATP hydrolysis.J. Biol. Chem. 258:7948–7953
Borlinghaus, R., Apell, H.-J., Läuger, P. 1987. Fast charge translocations associated with partial reactions of the Na/K-pump. I. Current and voltage transients after photochemical release of ATP.J. Membrane Biol. 97:161–178
Boyer, P.D. 1988. Bioenergetic coupling to protonmotive force: Should we be considering hydronium ion coordination and not group protonation?Trends Biochem. Sci. 12:5–7
Brahm, J. 1977. Temperature-dependent changes of chloride transport kinetics in human red cells.J. Gen. Physiol. 70:283–306
Cabantchik, Z. I., Knauf, P. A., Rothstein, A. 1978. The anion transport system of the red blood cell. The role of the membrane protein evaluated by the use of probes.J. Membrane Biol. 15:239–302
Cornelius, F., Skou, J.C. 1985. Na+−Na+ exchange mediated by (Na++K+)-ATPase reconstituted into liposomes. Evaluation of pump stoichiometry and response to ATP and ADP.Biochim. Biophys. Acta. 818:211–221
De Weer, P., Gadsby, D.C., Rakowski, R.F. 1988. Stoichiometry and voltage dependence of the sodium pump.In: The Na/K-Pump. J.C. Skou, J.G. Norby, A.B. Maunsbach, and M. Esmann, editors. Part A, pp. 421–434. A. R. Liss, New York
De Weer, P., Rakowski, R.F., Gadsby, D.C. 1987. Current-voltage relationships for the electrogenic sodium pump of squid axon.Biophys. J. 51:385a
Dissing, S., Hoffman, J.F. 1983. Anion-coupled Na efflux mediated by the Na/K pump in human red cells.Curr. Top. Membr. Transp. 19:693–695
Dixon, J.F., Hokin, L.E. 1980. The reconstituted (Na,K)-ATPase is electrogenic.J. Biol. Chem. 255:10681–10686
Eisner, D.A., Lederer, W.J. 1980. Characterisation of the electrogenic sodium pump in cardiac Purkinje fibres.J. Physiol. (London) 303:441–474
Eisner, D.A., Valdeolmillos, M., Wray, S. 1987. The effects of membrane potential on active and passive Na transport inXenopus oocytes.J. Physiol. (London) 385:643–659
Erdmann, E., Hasse, W. 1975. Quantitative aspects of ouabain binding to human erythrocytes and cardiac membranes.J. Physiol. (London) 251:671–683
Fendler, K., Grell, E., Haubs, M., Bamberg, E. 1985. Pump currents generated by the purified Na+, K+-ATPase from kidney on black lipid membranes.EMBO J.4:3079–3085
Forbush, B., III. 1987. Rapid release of42K and86Rb from an occluded state of the Na,K-Pump in the presence of ATP or ADP.J. Biol. Chem. 262:11104–11115
Forgac, M., Chin, G. 1982. Na transport by the (Na+)-stimulated adenosine triphosphatase.J. Biol. Chem. 257:5652–5655
Gadsby, D.C. 1984. The Na/K-pump of cardiac cells.Annu. Rev. Biophys. Bioeng. 13:373–398
Garrahan, P.J., Glynn, I.M. 1967a. The sensitivity of the sodium pump to external sodium.J. Physiol. (London) 192:175–188
Garrahan, P.J., Glynn, I.M. 1967b. The stoichiometry of the sodium pump.J. Physiol. (London) 192:217–235
Glynn, I.M. 1984. The electrogenic sodium pump.In: Electrogenic Transport. Fundamental Principles and Physiological Implications. M.P. Blaustein and M. Liberman, editors. Vol. 38, pp. 33–48. Society of General Physiologists Series
Glynn, I.M., Hoffman, J.F. 1971. Nucleotide requirements for sodium-sodium exchange catalyzed by the sodium pump in human red cells.J. Physiol. (London) 218:239–256
Glynn, I.M., Karlish, S.J.D. 1976. ATP hydrolysis associated with an uncoupled sodium flux through the sodium pump: Evidence for allosteric effects of intracellular ATP and extracellular sodium.J. Physiol. (London) 256:456–496
Goldshleger, R., Karlish, S.J.D., Rephaeli, A., Stein, W.D., 1987a. The effect of membrane potential on the mammalian sodium-potassium pump reconstituted into phospholipid vesicles.J. Physiol. (London) 387:331–355
Goldshleger, R., Karlish, S.J.D., Shahak, Y. 1987b. Electrogenic and electroneutral transport modes of the mammalian renal Na/K-pump.J. Physiol. (London) 390:98P
Hara, Y., Nakao, M. 1986. ATP-dependent proton uptake by proteoliposomes reconstituted with purified Na/K-ATPase.J. Biol. Chem. 261:12655–12658
Hoffman, J.H., Kaplan, J.H., Callahan, T.J. 1979. The Na∶K pump in red cells is electrogenic.Fed. Proc. 38:2440–2441
Jorgensen, P.L. 1974. Purification and characterization of (Na++K+) ATPase. III. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate.Biochim. Biophys. Acta. 356:53–67
Karlish, S.J.D. 1988a. Charge transfer by the Na/K-pump.In: The Na/K-Pump. J.C. Skou, J.G. Norby, A.B. Maunsbach, and M. Esmann, editors. Part A, pp. 519–524. A. R. Liss, New York
Karlish, S.J.D. 1988b. Measurement of active and passive Na+ and K+ fluxes in reconstituted vesicles.Methods Enzymol. 156:179–188
Karlish, S.J.D., Lieb, W.R., Stein, W.D. 1982. Combined effects of ATP and phosphate on rubidium exchange mediated by Na−K-ATPase reconstituted into phospholipid vesicles.J. Physiol. (London) 328:333–350
Karlish, S.J.D., Pick, U. 1981. Sidedness of the effects of sodium and potassium ions on the conformational state of the sodium-potassium pump.J. Physiol. (London) 312:505–529
Karlish, S.J.D., Stein, W.D. 1982a. Effects of ATP or phosphate on passive rubidium fluxes mediated by Na−K-ATPase reconstituted into phospholipid vesicles.J. Physiol. (London) 328:317–331
Karlish, S.J.D., Stein, W.D. 1982b. Passive rubidium fluxes mediated by Na−K-ATPase reconstituted into phospholipid vesicles when ATP- and phosphate-free.J. Physiol. (London) 328:295–316
Karlish, S.J.D., Stein, W.D. 1985. Cation activation of the pig kidney sodium pump: Transmembrane allosteric effects.J. Physiol. (London) 359:119–149
Navarro, J., Essig, A. 1984. Voltage-dependence of Ca2+ uptake and ATP hydrolysis of reconstituted Ca-ATPase vesicles.Biophys. J. 46:709–717
Penefsky, H.S. 1977. Reversible binding of Pi by beef heart mitochondria adenosine triphosphatase.J. Biol. Chem. 252:2891–2899
Polvani, C., Blostein, R. 1988. Protons as substitutes for sodium and potassium in the sodium pump reaction.J. Biol. Chem. 263:16757–16763
Polvani, C., Blostein, R. 1989. Effect of cytoplasmic sodium concentration on the electrogenicity of the sodium pump.J. Biol. Chem. 264:15182–15185
Post, R.L., Jolly, P.C. 1957. The linkage of sodium, potassium and ammonium active transport across the human erythrocyte membrane.Biochim. Biophys. Acta. 25:118–128
Schuurmans, J.J., Casey, R.P., Kraayenhof, R. 1978. Transmembrane electrical potential formation in spinach chloroplasts: Investigation using a rapidly-responding extrinsic probe.FEBS Lett 94:405–409
Shahak, Y., Admon, A., AVron, M. 1982. Transmembrane electrical potential formation in chloroplast (CF1−CF0) proteoliposomes.FEBS Lett. 150:27–31
Shani, M., Goldshleger, R., Karlish, S.J.D. 1987. Rb occlusion in renal (Na,K)ATPase characterised with a simple manual assay.Biochim. Biophys. Acta. 904:13–21
Shani-Sekkler, M., Goldshleger, R., Tal, D., Karlish, S.J.D. 1988. Inactivation of Rb+ and Na+ occlusion on (Na+, K+)-ATPase by modification of carboxyl groups.J. Biol. Chem. 263:19331–19342
Shanzer, A., Samuel, D., Korenstein, R. 1983. Lipophilic lithium ion carriers.J. Am. Chem. Soc. 105:3815–3818
Smith, J.C., Chance, B. 1979. Kinetics of the potential-sensitive extrinsic probe oxonol VI in beef heart sub-mitochondrial particles.J. Membrane Biol 46:255–282
Thomas, R.C. 1972. Electrogenic sodium pump in nerve and muscle cells.Physiol. Rev. 52:563–594
Thomas, R.C. 1984. Electrogenic sodium pump current associated with recovery from intracellular acidification of snail neurones.In: Electrogenic Transport. Fundamental Principles and Physiological Implications. M.P. Blaustein, and M. Lieberman, editors. Vol. 38, pp. 3–16. Society of General Physiologists Series
Waggoner, A.S. 1985. Dye probes of cell, organelle and vesicle membrane potentials.In: The Enzymes of Biological Membranes. A.N. Martonosi, editor. Vol. 3, pp. 313–331. Plenum Press, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goldshleger, R., Shahak, Y. & Karlish, S.J.D. Electrogenic and electroneutral transport modes of renal Na/K ATPase reconstituted into proteoliposomes. J. Membrain Biol. 113, 139–154 (1990). https://doi.org/10.1007/BF01872888
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01872888