Abstract
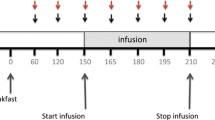
The effects of the intraduodenal administration of a low dose of CR-1505 for 3–7 days on the gene expression of cholecystokinin (CCK), plasma CCK concentration, and CCK content in the intestinal mucosa were examined in rats. The simultaneous changes of protein and enzyme content in the pancreas were also determined. CR-1505 was infused continuously into the duodenum at a dose of 3 mg/kg per day, calculated to correspond to a dose of 150–200 mg/day in humans. Seven days after the administration of CR-1505, a liquid meal (4.5 kcal/3 ml) was introduced into the stomach and changes in the intestinal CCK content and plasma CCK concentration were examined. The level of CCK mRNA in the intestine was significantly higher in rats treated with CR-1505 than in control rats. The plasma CCK concentration, the CCK content of the intestinal mucosa, and the composition of pancreatic enzymes did not significantly differ in rats treated with CR-1505 and the untreated controls. In control rats, the administration of the liquid meal increased the plasma CCK concentration and significantly decreased the intestinal CCK content in water extracts, but did not affect the amount extracts in acid whereas the ingestion of the meal did not cause any significant changes in rats treated with CR-1505. These findings indicate that a low dose of CR-1505 stimulates the gene expression of CCK without enhancing CCK release or exerting an effect on the pancreas.
Similar content being viewed by others
References
Johnson LR. Pancreatic secretion. In: Johnson LR (ed) Gastrointestinal physiology, St. Louis: Mosby, 1985;83–93.
Mainz DL, Black O, Wester PD Hormonal control of pancreatic growth. J Clin Invest 1973 52:2300–2394.
Barrowman JA, Mayston PA. The trophic influence of cholecystokinin on the rat pancreas. J Physiol 1974; 238:73–75.
Robert TJ, Zhi-Chao Z, Randall BM, et al. Structural features of various proglumide-related cholecystokinin receptor antagonisis. Am J Physiol 1986; 251:G839-G845.
Jansen RT, Murphy RB, Trampota M, et al. Proglumide analogue: Potent cholecystokinin receptor antagonists. Am J Physiol 1985; 249:G214-G220.
Tani Y, Baba N, Nishimura I, et al. Effects of CR1505, a CCK antagonist, on pancreatic growth in hamsters. Jpn J Gastroenterol 1991; 88:1097–1104.
Göke B, Printz H, Koop I, et al. Endogenous CCK release and pancreatic growth in rats after feeding a protease inhibitor (camostate). Pancreas 1986; 1:509–515.
Niederau C, Liddle RA. Williams TA. Pancreatic growth: Interaction of exogenous cholecystokinin, a protease inhibitor, and a cholecystokinin receptor antagonist in mice. Gut 1987; 28:63–69.
Suzuki M, Kanno T Eutrophic effect of endogenous CCK on digestive enzymes in the rat pancreas (in Japanese). In: Miyoshi A (ed) Shoukakann Hormone (IX) Tokyo: Igakutosho shuppan, 1990;467–473.
Schmidt WE, Creutzfeldt W, Höcker M, et al Cholecystokinin receptor antagonist Loxiglumide: Inflence on bile-pancreatic secretion and gastrointestinal hormones in man. Digestion 1990;46:232–239.
Funakoshi A, Miyasaka K. Significance of cholecystokinin and progression of chronic pancreatitis. Gastroenterologia Jpn 1993;28:601.
Funakoshi A, Miyasaka K, Kawanami T, et al. Effect of a CCK antagonist (CR 1505) on gene expressions of cholecystokinin and secretin in rat intestine. Pancreas 1995;10:118–122.
Liddle RA, Goldfine ID, Williams JA, Bioassay of plasma cholecystokinin in rats: Effects of food, trypsin inhibitor, and alcohol. Gastroenterology 1984;87:542–549.
Nakamura R, Miyasaka K, Funakoshi A, Kitani K. Interactions between bile and pancreatic juice diversions on cholecystokinin release and pancreas in conscious rats. Proc Soc Exp Biol Med 1989;192:182–186.
Kanayama S, Liddle RA. Influence of food deprivation on intestinal cholecystokinin and somatostatin. Gastroenterology 1991;100:909–915.
Funakoshi A, Tanaka A, Tateishi K, et al. Different expression of the cholecystokinin (CCK) precursor gene in rat tissue. J Gastroenterol 1994;29:125–128.
Miyasaka K, Funakoshi A, Jimi A, et al. Changes in plasma and duodenal cholecystokinin concentrations after pancreatic occlusion in rats. Dig Dis Sci 1992;37:369–377.
Tateishi K, Funakoshi A, Jimi A, et al. High plasma pancreastatin-like immunoreactivity in a patient with malignant insulinoma. Gastroenterology 1989;97:1313–1318.
Funakoshi A, Nakano I, Shinozaki H, et al. High plasma cholecystokinin levels in patients with chronic pancreatitis having abdominal pain. Am J Gastroenterol 1986;81:1174–1178.
Miyasaka K, Kitani K. Aging impairs pancreatic response to refeeding following a protein-free diet. Pancreas 1989; 4:346–352.
Miyasaka K, Nakamura R, Kitani K. Effects of trypsin inhibitor (camostate) on pancreas and CCK release in young and old female rats. J Gerontol. 1989;44:136–140.
Walsh KA, Wilcox PE. Serine proteases. Methods Enzymol 1970;19:31–41.
Ceska M, Birath K, Brown B. A new and rapid method for the clinical determination of α-amylase activities in human serum and urine. Clin Chim Acta 1969;26:437–444.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265–275.
Jansen JBM, Jong AJL, Lamers C. The cholecystokinin receptor antagonist CR 1409 increases plasma cholecystokinin in rats. Regul Pept 1989;27:209–213.
Green GM, Lyman RL. Feedback regulation of pancreatic enzyme secretion as a mechanism for trypsin inhibitor produced hypersecretion in rats. Proc Soc Exp Biol Med 1972;104:6–12.
Fölsch UR, Cantor P, Wilms HM, et al. Role of cholecystokinin in the negative feedback control of pancreatic enzyme secretion in conscious rats. Gastroenterology 1987;92:449–458.
Louie DS, May D, Miller P, Owyang C Cholecystokinin mediates feedback regulation of pancreatic enzyme secretion in rats. Am J Physiol 1986;250:G252–259.
Miyasaka K, Guan D, Liddle A, Green GM. Feedback regulation by Grypsin: Evidence for intraluminal CCK-releasing peptide. Am J Physiol 1983;257:G175-G181.
Funakoshi A, Miyasakt K. Effect of a new CCK antagonist (FK 480) on gene expressions oa cholecystokinin and secretin in rat intestine. J Gastroenterol 1994;29:385–387.
Moran TH, Norgern R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res 1990;526:95–102.
Moran TH, Smith GP, Hostetler AM, McHugh PR. Transport of cholecystokinin (CCK) binding sites in subdiaphragmatic vagal branches. Brain Res 1987;415:149–152.
Merger JG, Lawrence CB, Morgan PJ. G-protein coupling of vagal CCK binding sites and comparisons of transport rates. Physiol Behav 1993;53:1061–1065.
Li Y, Owyang C. Vagal afferent pathway mediates physiological action of cholecystokinin on pancreatic enzyme secretion. J Clin Invest 1993;92:418–424.
Moran TH, McHugh PR. Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am J Physiol 1982;242:R491-R497.
Tomita H, Miyasaka K, Jimi A, et al. Lack of effect of cholecystokinin receptor antagonist (CR 1505) on recovery of experimental pancreatitis after pancreatic duct occlusion in rats. Pancreas 1994;9:638–645.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sazaki, N., Miyasaka, K., Matsumoto, M. et al. Effects of intraduodenal administration of a low dose of cholecystokinin (CCK) antagonist (CR-1505) on plasma CCK concentration, intestinal CCK content, and levels of CCK mRNA. J Gastroenterol 30, 599–606 (1995). https://doi.org/10.1007/BF02367785
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02367785