Abstract
Background
To investigate the mechanism of glycerol-induced renal injury, we examined kidney levels of the cellular antioxidant glutathione and heme oxygenase-1 (HO-1), the rate-limiting enzyme in heme degradation, after glycerol injection.
Methods
Male Wistar rats were injected with glycerol to induce acute renal failure. Serum creatinine levels were used as a marker of renal function at 24 hours after glycerol injection. Theophylline and buthionine sulfoximine or vehicle were administered to the rats after glycerol injection, and we determined renal glutathione levels (by biochemical assay) and the levels of HO-1 protein and messenger ribonucleic acid (mRNA; using immunoblot analysis [kidney only]) in various rat organs at 24 hours after glycerol injection.
Results
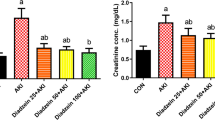
Glutathione levels abruptly declined after glycerol injection, reached a minimum at 4 hours, then returned to about two thirds of control levels at 24 hours. HO-1 protein was detected at 4 hours and reached a maximum at 24 hours. The induction of HO-1 protein was observed only in the kidney. HO-1 mRNA was faintly expressed at 2 hours, increased until at least 8 hours, and was not detected 24 hours after the treatment. When theophylline was administered to glycerol-injected rats, renal function improved and glutathione levels increased. In addition, the levels of HO-1 protein decreased, compared with those of glycerol-treated rats not given theophylline.
Conclusions
These results suggest that theophylline may act by modulating the HO-1 directly or indirectly.
Similar content being viewed by others
References
Shah SV, Walker PD, Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol 1989;255:F438-F443.
Vogt BA, Alam J, Croatt AJ, Vercellotti GM, Nath KA. Acquired resistance to acute oxidative stress. Possible role of heme oxygenase and ferritin. Lab Invest 1995;72:474–483.
Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol 1988;255:F539-F544.
Tappel AL. The mechanism of the oxidation of unsaturated fatty acids catalyzed by hematin compounds. Arch Biochem Biophys 1953;44:378–395.
Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin bound bilirubin. Proc Natl Acad Sci USA 1987;84:5918–5922.
Aust SD, Svingen BA. The role of iron in enzymatic lipid peroxidation. In: Pryor WA (ed) Free radicals in biology. New York: Academic Press, 1982;5:1–28.
Brüne B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol 1987;32:497–504.
Furchgott RF, Jothiananan D. Endothelium-dependent and-independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels 1991;28:52–61.
Ewing JF, Rajn VS, Maines MD. Induction of heart heme oxygenase-1 (HSP32) by hyperthermia possible role in stress-mediated elevation of cyclic 3′∶5′-guanosine monophosphate. J Pharmacol Exp Ther 1994; 271:408–414.
Prabhaker NR, Dinerman JL, Agani FH, Snyder SH. Carbon monoxide: a role in carotid body chemoreception. Proc Natl Acad Sci USA 1995;92:1994–1997.
Johnson RA, Lavesa M, Askari B, Abraham NG, Nasjletti A. A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension 1995;25:166–169.
Abul-ezz SR, Walker PD, Shah SV. Role of glutathione in an animal model of myoglobinuric acute renal failure. Proc Natl Acad Sci USA 1991;88:9833–9837.
Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 1988;2:2557–2568.
Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA-radiation, hydrogen peroxide and sodium arsenite. Proc Natl Acad Sci USA 1989;85:99–103.
Tomaro ML, Frydman J, Frydman RB. Heme oxygenase induction by CoCl2, co-protoporphyrin IX, phenylhydrazine, and diamide: evidence for oxidative stress involvement. Arch Biochem Biophys 1991;286:610–617.
Heidemann HT, Müller S, Mertins L, Stepan G, Hoffmann K. Effect of aminophylline on cisplatin nephrotoxicity in the rat. Br J Pharmacol 1989;97:313–318.
Meister A. Selective modification of glutathione metabolism. Science 1983;220:472–477.
Meister A. New developments in glutathione metabolism and their potential application in therapy. Hepatology 1984;4:739–742.
Meister A, Anderson ME, Hwang O. Intracellular cysteine and glutathione delivery systems. J Am Coll Nutr 1986;5:37–151.
Olson CE. Glutathione modulates toxic oxygen metabolite injury of canine chief cell monolayers in primary culture. Am J Physiol 1988;254:G49-G56.
Arrick BA, Nathan CF, Griffith OW, Cohn ZA. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem 1982;257:1231–1237.
Hue DP, Griffith KL, McLean A. Hepatocytes in primary culture become susceptible to paracetamol injury after depletion of glutathione using DL-buthionine-SR-sulphoximine. Biochem Pharmacol 1985;34:4341–4344.
Andreoli SP, Mallett CP, Bergstein JM. Role of glutathione in protecting endothelial cells against hydrogen peroxide oxidant injury. J Lab Clin Med 1986; 108:190–198.
Suttorp N, Toepfer W, Roka L. Antioxidant defense mechanisms of endothelial cells: glutathione redox cycle versus catalase. Am J Physiol 1986;251:5:C671–680.
Tietze F. Enzymic method for quantitative determination of nanogram amount of total and oxidized glutathione: application to mammalian blood and other tissues. Anal Biochem 1969;27:502–522.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
Laemmli UK. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 1970;227:680–685.
Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Anal Biochem 1987; 162:156–159.
Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor NY; Spring Harbor Laboratory, 1989.
Shibahara S, Muller R, Taniguchi H, Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc Natl Acad Sci USA 1985;82:7865–7869.
Ayer G, Grandchamp A, Wyler T, Truniger B. Intrarenal hemodynamics in glycerol induced myohemoglobinuric acute renal failure in the rat. Cir Res 1971;29:128–135.
Gould J, Bowmer CG, Yates MS. Renal hemodynamic responses to adenosine in acute renal failure. Nephron 1995;71:184–189.
Franco M, Bell PD, Navar LG. Effect of adenosine A1 analogue on tubuloglomerular feedback mechanism. Am J Physiol 1989;257:F231-F236.
Karam H, Bruneval P, Clozel JP, Liffler BM, Barity J, Clozel M. Role of endothelin in acute renal failure due to rhabdomyolysis in rats. J Pharm Exp Ther 1989; 274:481–486.
Agarwal A, Balla J, Alam J, Croatt A J, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int 1995;48:1298–1307.
Guidet B, Shah SV. Enhanced in vivo H2O2 generation by rat kidney in glycerol-induced renal failure. Am J Physiol 1989;257:F440-F445.
Meister A. Metabolism and function of glutathione: an overview. Biochem Soc Trans 1982;10:78–79.
Llesuy SF, Tomaro ML. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta 1994;1223:9–14.
Christodoukides N, Durante W, Kroll MH, Schafer AI. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase stimulatory carbon monoxide. Cir Res 1995;91:2306–2309.
Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem 1986; 261:411–419.
Keyse SM, Tyrrell RM. Both near ultraviolet radiation and the oxidizing agent hydrogen peroxide induce a 32-kDa stress protein in normal human skin fibroblast. J Biol Chem 1987;262:14821–14825.
Nath KA, Vercellotti GM, Balla J, Jacob HS, Lavitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 1992;90:267–270.
Linn JJ, Churchill PC, Bidni AK. Theophylline in rats during maintenance phase of post ischemic acute renal failure. Kidney Int 1988;33:24–28.
Aoyagi K, Nagase S, Narita M, Tojo S. Role of active oxygen on methylguanidine synthesis in isolated rat hepatocytes. Kidney Int 1987;32(suppl 22):S229-S233.
Sardana MK, Sassa S, Kappas A. Hormonal regulation of heme oxygenase induction in avian hepatocyte culture. Biochem Pharmacol 1985;34:2937–2944.
Author information
Authors and Affiliations
About this article
Cite this article
Ishizuka, S., Nagashima, Y., Sone, M. et al. Theophylline protects against glycerol-induced acute renal failure: Regulation of heme oxygenase-1 and glutathione in rat kidney. Clin Exper Neph 1, 204–211 (1997). https://doi.org/10.1007/BF02480696
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02480696