Summary
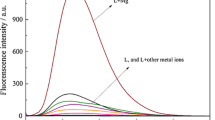
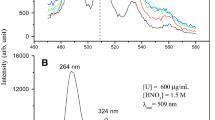
A fluorimetric method for the determination of Mg(II), based on the formation of a fluorescent 1∶1 chelate with 1,8-dihydroxyanthraquinone, is described. With 90% ethanol in ammoniacal medium, the fluorescent complex has excitation maxima at 470, 480 and 495 nm and emission maximum at 600 nm. The logarithm of apparent formation constant of the complex is 4.68. The calibration graph is linear over the range 5–350 ppb Mg(II).
Zusammenfassung
Die Methode beruht auf der Bildung eines fluoreszierenden 1∶1-Chelates von Mg(II) mit 1,8-Dihydroxyanthrachinon. In ammoniakalischem, 90% Ethanol enthaltendem Medium hat der fluoreszierende Komplex Anregungsmaxima bei 470, 480 und 495 nm und ein Emissionsmaximum bei 600 nm. Der Logarithmus der scheinbaren Bildungskonstante des Komplexes beträgt 4,68. Die Eichkurve verläuft zwischen 5 und 350 ppb Mg(II) linear.
Similar content being viewed by others
References
H. Gillet and J. Ch. Pariaud, Bull. soc. chim. France8, 2624 (1966).
M. Román, A. Fernández-Gutiérrez, and M. C. Mahedero, An. Quim.74, 3 (1978).
M. Román, A. Arrebola, and D. V. González, Quim. Anal.31 (2), 91 (1977).
C. E. White and C. S. Lowe, Ind. Eng. Chem., Analyt. Ed.13, 809 (1941).
I. M. Koremann, B. A. Nikolaev, and G. B. Smakov, Tr. Po. Khim. Teknol.2, 406 (1962).
R. Ruggieri, Analyt. Chim. Acta25, 145 (1961).
H. Gillet, C. acad. sci., Paris, Ser. C266 (10), 683 (1968).
Y. Asahi, K. Shinozaki, M. Mitainy, and H. Ohtsuka, Chem. Pharm. Bull. Tokyo22 (2), 254 (1974).
M. Ashraj and J. B. Headridge, Talanta16 (10), 1439 (1969).
D. Schachter, J. Lab. Clin. Med.54 (5), 763 (1959).
D. Schachter, J. Lab. Clin. Med.58, 495 (1961).
D. P. Shcherbov, R. N. Plotnikova, S. A. Voinovov, Prom. Khim. Reaktivov Osobo Chist. Veshchestv, no. 8, 166 (1967).
A. Badrinas, Talanta10, 74 (1963).
G. P. Gusev, Lab. Delo,3, 157 (1968).
G. V. Serebryakova, A. M. Lukin, E. A. Bozhevol'nov, U. S. S. R. 129, 273, June 15 (1960); Chem. Abstr.55, 2365 a (1961).
G. V. Serebryakova, A. M. Lukin, and E. A. Bozhevol'nov, Zh. Anal. Khim.18, 706 (1963).
R. M. Dagnall, R. Smith, and T. S. West, Analyst92, 20 (1967).
T. Hayashi, S. Kawai, and T. Ohno, Chem. Pharm. Bull. (Tokyo)18, 2407 (1970).
A. Korkuc and K. Lesz, Chem. Anal.17 (4), 855 (1972).
K. Kasiura, Chem. Anal.20, 389 (1975).
C. E. White and F. Cuttita, Analyt. Chemistry31, 2083 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Román Ceba, M., Fernández-Gutiérrez, A. & Mahedero, M.C. Fluorimetric determination of Mg(II) traces with 1,8-dihydroxyanthraquinone. Mikrochim Acta 80, 85–94 (1983). https://doi.org/10.1007/BF01204586
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01204586