Abstract
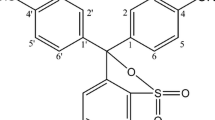
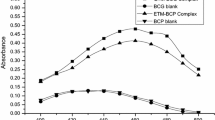
The reagent, ethylisobutrazine hydrochloride, reacts with osmium (VIII) or osmium (VI) to form a red complex species instantaneously in 2M hydrochloric acid medium at room temperature, 25±2°C. The complex exhibits absorption maximum around 519 nm. Based on this complexation reaction, a rapid, sensitive and selective Spectrophotometric method is developed for the determination of osmium. The Beer's law is valid over the concentration range 0.25–7.5 ppm for osmium(VIII) and 0.25–8.0 ppm for osmium(VI). The molar absorptivities are 2.4×104 1 mol−1 cm−1 and 1.8×104 1 mol−1 cm−1 for osmium (VIII) and osmium(VI), respectively. The effects on the reaction of experimental conditions, such as time, temperature, acidity, order of addition of reactants, reagent concentration, and diverse ions, are reported. The sensitivity of the proposed method, as evaluated from the Beer's law data, was 7.0 ng cm−2 for osmium (VIII) or 9.2 ng cm−2 for osmium(VI).
The analyses of sample solutions containing 2.0 ppm of osmium (VIII) and 3.0 ppm of osmium(VI) showed that the recovered amounts of the analytes had relative standard deviations of 0.0018 and 0.0022 ppm, respectively, for ten replicate determinations. The method has been successfully applied to the determination of osmium content in the synthetic syserkite mineral.
Similar content being viewed by others
References
F. E. Beamish, J. C. Van Loon,Recent Advances in the Analytical Chemistry of the Noble Metals, Pergamon Press, Oxford, 1972, p. 306.
F. E. Beamish,The Analytical Chemistry of the Noble Metals, Pergamon Press, Oxford, 1966, p. 363.
F. E. Beamish,Talanta,1965,12, 789.
D. F. Botz, M. G. Mellon,Anal. Chem. 1976,48, 216R;1974,46, 234R;1972,44, 300R;1970,42, 152R.
J. A. Howell, L. G. Hargis,Anal. Chem. 1982,54, 171R;1980,52, 306R;1978,50, 243R.
G. Goldstein, O. L. Manning, O. Menis, J. A. Dean,Talanta 1961,7, 301.
S. Kalyanaraman, S. M. Khopkar,Anal. Chim. Acta 1975,78, 231.
B. V. Agarwal, A. K. Ghose,Talanta 1973,20, 129.
G. H. Ayres, W. N. Wells,Anal. Chem. 1950,22, 317.
E. A. Klobbie,Chem. Zbl. 1898,11, 65.
P. C. Dwivedi, S. N. Gurudath Rao, S. N. Bhat, C. N. R. Rao,Spectrochim. Acta 1975,31A, 129.
I. S. Forrest, F. M. Forrest, M. Berger,Biochim. Biophys. Acta 1958,29, 441.
M. Stan, V. Dorneanu, G. H. Ghimicescu,Talanta 1977,24, 140.
A. Ringbom,Z. Anal. Chem. 1939,115, 332.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thimme Gowda, A., Made Gowda, N.M. Spectrophotometric determination of osmium with ethylisobutrazine hydrochloride. Mikrochim Acta 88, 351–357 (1986). https://doi.org/10.1007/BF01206729
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01206729