Summary
Four strains of potato virus Y, PVY-D, PVY-10, PVY-18, and PVY-43, obtained from different Australian sources were compared on the basis of their biological, serological and coat protein structural properties. Each of the strains could be distinguished on the basis of their reactions on selected test plant species. Two of the PVY strains, PVY-D and PVY-10, induced symptoms similar to those produced by the PVYO strain group. The reactions of PVY-18 and PVY-43, although comparable to PVYN in some hosts, did not completely match the description of the PVYN strain group. In contrast to the other three strains, PVY-18 could not be transmitted byMyzus persicae in repeated tests. No difference was observed in the serological properties of the four PVY strains in different assay systems, using polyclonal antisera.
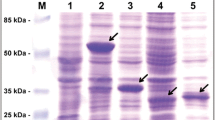
The amino acid sequences of the coat proteins of PVY-10, PVY-18, and PVY-43 were obtained and compared with the coat protein sequences of pepper mottle virus (PeMV) [Dougherty WG, Allison RF, Parks TD, Johnston RE, Feild MJ, Armstrong FB (1985) Virology 146: 282–292] and PVY-D [Shukla DD, Inglis AS, McKern NM, Gough KH (1986) Virology 152: 118–125]. The homology between the PVY strains ranged from 96.3 to 99.3% and with the PeMV sequence, 91.4 to 92.9%. Based on this high sequence homology, and the previous observation that coat protein sequences of potyvirus strains are always greater than 90% identical, PeMV could be considered a strain of PVY. However, PVY and PeMV are reported to be only distantly serologically related and on this basis PeMV is currently considered to be an independent member of the Potyvirus group.
Similar content being viewed by others
References
Allison RF, Dougherty WG, Parks TD, Willis L, Johnston RE, Kelly ME, Armstrong FB (1985) Biochemical analysis of capsid protein gene and capsid protein of tobacco etch virus: N-terminal amino acids are located on the virion's surface. Virology 147: 309–316
Bos L (1979) The identification of three new viruses isolated fromWisteria andPisum in the Netherlands, and the problems of variation within the potato virus Y group. Neth J Plant Pathol 76: 8–46
Burk LG, Gooding GV Jr, Chaplin JF (1982) Reaction ofNicotiana species and cultivars or breeding lines ofNicotiana tabacum to three strains of potato virus Y. Tobacco Sci 26: 85–88
Clark MF, Adams AN (1977) Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol 34: 475–483
De Bokx JA, Huttinga H (1981) Potato virus Y. CMI/AAB Descript Plant Vir 242
De Bokx JA, Krachanova B, Maat DZ (1975) Some properties of a deviating strain of potato virus Y. Potato Res 18: 38–51
Dougherty WG, Allison RF, Parks TD, Johnston RE, Feild MJ, Armstrong FB (1985) Nucleotide sequence of the 3′ terminus of pepper mottle virus genomic RNA: evidence for an alternative mode of potyvirus capsid protein gene organization. Virology 146: 282–291
Evans IR, Zettler FW (1970) Aphid and mechanical transmission properties of bean yellow mosaic virus isolates. Phytopathology 60: 1170–1174
Francki RIB (1983) Current problems in plant virus taxonomy. In: Matthews REF (ed) A critical appraisal of viral taxonomy. CRC Press, Boca Raton, pp 63–104
Francki RIB, Milne RG, Hatta T (1985) Atlas of plant viruses, vols 1 and 2. CRC Press, p 284
Gooding GV Jr, Tolin SA (1973) Strains of potato virus Y affecting flue-cured tobacco in the south-eastern United States. Plant Dis Rep 57: 200–204
Gough KH, Shukla DD (1981) Coat protein of potyviruses. 1. Comparison of the four Australian strains of sugarcane mosaic virus. Virology 111: 455–462
Gugerli P, Fries P (1983) Characterization of monoclonal antibodies to potato virus Y and their use for virus detection. J Gen Virol 64: 2471–2477
Harrison BD (1985) Usefulness and limitations of the species concept for plant viruses. Intervirology 25: 71–78
Heath R, Sward RJ, Moran JR, Mason AJ, Hallam ND (1987) Biological characterization of six Australian isolates of potato virus Y. Aust J Agric Res 38: 395–402
Hewish DR, Shukla DD, Gough KH (1986) The use of biotin-conjugated antisera in immunoassay for plant viruses. J Virol Methods 13: 79–85
Hollings M, Brunt AA (1981) Potyvirus group. CMI/AAB Descript Plant Vir 245
Hollings M, Brunt AA (1981) Potyviruses. In: Kurstak E (ed) Handbook of plant virus infections: comparative diagnosis. Elsevier/North Holland Amsterdam, pp 731–807
Horvath J (1967) Studies on strains of potato virus Y. 4. Anomolous strains. Acta Phytopathol Acad Sci Hung 2: 195–210
Lattore BA, Andrade O, Penaloza E, Escaffi O (1982) Severe outbreak of potato virus Y in Chilean tobacco. Plant Dis 66: 893–895
Leiser RM, Richter J (1979) Ein Beitrag zur Frage der Differenzierung von Stämmen des Kartoffel-Y-Virus. Arch Phytopathol Pfl Schutz 15: 289–298
Matthews REF (1982) Classification and nomenclature of viruses. Fourth report of the international committee on taxonomy of viruses. Intervirology 17: 1–199
Nelson MR, Wheeler RE (1978) Biological and serological characterization and separation of potyviruses that infect peppers. Phytopathology 68: 979–984
O'Donnell IJ, Shukla DD, Gough KH (1982) Electro-blot radioimmunoassay of virus-infected plant sap — a powerful new technique for detecting plant viruses. J Virol Methods 4: 19–26
Park EK, Kim JJ, Boo KS (1984) Two new potato virus Y strains isolated from tobacco plants in Korea. Korean J Plant Prot 23: 209–214
Purcifull DE, Batchelor DL (1977) Immunodiffusion tests with sodium dodecyl sulfate (SDS) — treated plant viruses and plant virus inclusions. Fla Agric Exp Sta Bull No 788
Purcifull DE, Zitter TA, Hiebert E (1975) Morphology, host range and serological relationships of pepper mottle virus. Phytopathology 65: 559–562
Rose DG, Hubbard AL (1986) Production of monoclonal antibodies for the detection of potato virus Y. Ann Appl Biol 109: 317–321
Rose DG, McCarra S, Mitchel DH (1987) Diagnosis of potato virus YN: a comparison between polyclonal and monoclonal antibodies and a biological assay. Plant Pathol 36: 95–99
Shukla DD, Gough KH, Ward CW (1987) Coat protein of potyviruses. 3. Comparison of amino acid sequences of the coat proteins of four Australian strains of sugarcane mosaic virus. Arch Virol 96: 59–74
Shukla DD, Inglis AS, McKern NM, Gough KH (1986) Coat protein of potyviruses. 2. Amino acid sequence of coat protein of potato virus Y. Virology 152: 118–125
Shukla DD, Jilka J, Tosic M, Ford RE (1988) A novel approach to serology of potyviruses involving affinity purified polyclonal antibodies directed towards virus-specific N-termini of coat proteins. J Gen Virol, in press
Shukla DD, McKern NM, Ward CW (1988) Coat proteins of potyviruses. 5. Symptomatology, serology and coat protein sequences of three strains of passionfruit woodiness virus. Arch Virol 102: 221–232
Shukla DD, McKern NM, Gough KH, Tracy SL, Letho SG (1988) Differentiation of potyviruses and their strains by high performance liquid chromatographic peptide profiling of coat proteins. J Gen Virol 69: 493–502
Shukla DD, Strike PM, Tracy SL, Gough KH, Ward CW (1988) The N and C termini of the coat proteins of potyviruses are surface-located and the N terminus contains the major virus-specific epitopes. J Gen Virol 69: 1417–1508
Shukla DD, Ward CW (1988) Amino acid sequence homology of coat proteins as a basis for identification and classification of the potyvirus group. J Gen Virol, in press
Silberschmidt KM (1960) Types of potato virus Y necrotic to tobacco: history and recent observations. Am Potato J 37: 151–159
Simons JN (1976) Aphid transmission of a non-aphid-transmissible strain of tobacco etch virus. Phytopathology 66: 652–654
Thomas JE, McGrath DJ (1988) Inheritance of resistance to potato virus Y in tomato. Aust J Agric Res 39: 475–479
Thottapilly G, Tsai JR, Bath JE (1972) Differential aphid transmission of two bean yellow mosaic strains and comparative transmission by biotypes and stages of the pea aphid. Ann Entomol Soc Am 65: 912–915
Woolston CJ, Czaplewski LG, Markham PG, Goad AS, Hull R, Davies JW (1987) Location and sequence of a region of cauliflower mosaic virus gene 2 responsible for aphid transmissibility. Virology 160: 246–251
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shukla, D.D., Thomas, J.E., McKern, N.M. et al. Coat protein of Potyviruses. Archives of Virology 102, 207–219 (1988). https://doi.org/10.1007/BF01310826
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01310826