Summary
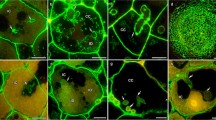
When vesicular-arbuscular mycorrhizal (VAM) fungi colonize the cortical cells of their host plant roots, the hyphae are separated from the host cytoplasm by the invaginated host plasmalemma and interfacial material. The presence of hydroxyproline rich glycoproteins (HRGPs) at the interface was investigated with a polyclonal antibody obtained against melon callus HRGP2b. By using a combination of cytochemical methods, antigens were detected in pea, in both the presence and absence ofGlomus versiforme, a mycorrhizal fungus. For comparison, observations were performed in parallel with leek as a monocot host. Antigens were localized over the pea cell wall in root tissues. At the ultrastructural level, gold granules were mostly present in the periplasmic space. In mycorrhizal plants, the most substantial deposition occurred at the interface between the fungal wall and the host membrane. Dot blot experiments revealed HRGP2b antigens in soluble root fractions from both uninfected and mycorrhizal samples.
The results demonstrate that HRGP2b antigens can be localized over the cell wall of both dicot and monocot hosts, although they mostly occur in the contact zone in infected samples. Their presence-in the company of localized glucans and pectins-means that the contact zone can be regarded as an apoplastic space presenting a structural response to the symbiotic mycorrhizal status.
Similar content being viewed by others
References
Benhamou N, Lafontaine PJ, Mazau D, Esquerré-Tugayé MT (1991) Differential accumulation of hydroxyproline-rich glycoproteins in bean root nodule cells infected with a wild type strain or a C4-dicarboxylic acid mutant ofRhizobium leguminosarum bv.phaseoli. Planta 184: 457–467
—, Mazau D, Esquerré-Tugayé MT, Asselin A (1990 a) Immunogold localization of hydroxyproline-rich glycoproteins in necrotic tissue ofNicotiana tabacum L. cv.Xanti-nc infected by tobacco mosaic virus. Physiol Mol Plant Pathol 36: 129–145
— — —, (1990 b) Immunocytochemical localization of hydroxy-proline-rich glycoproteins in tomato root cells infected byFusarium oxisporum f. sp.radicis-lycopersici: study of a compatible interaction. Pythopathology 80: 163–173
Biggs KJ, Fry SC (1990) Solubilization of covalently bound extensin fromCapsicum cell walls. Plant Physiol 92: 197–204
Bonfante-Fasolo P (1984) Anatomy and morphology. In: Powell CL, Bagyaraj DJ (eds) V.A. mycorrhizas. CRC Press, Boca Raton, pp 5–33
Bonfante-Fasolo P, Perotto S (1990) Mycorrhizal and pathogenic fungi: do they share any features? In: Mendgen K, Lesemann DE (eds) Electron microscopy applied in plant pathology. Springer, Berlin Heidelberg New York Tokyo, pp 265–275
—, Vian B, Perotto S, Faccio A, Knox JP (1990) Cellulose and pectin localization in roots of mycorrhizalAllium porrum: labelling continuity between host cell wall and interfacial material. Planta 180: 537–547
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72: 248–254
Cooper JB (1984) Hydroxyproline synthesis is required for cell wall regeneration. In: Dugger WM, Bartnicki-Garcia S (eds) Structure, function, and biosynthesis of plant cell walls. Waverly Press, Baltimore, MD, pp 397–399
—, Chen JA, vanHolst GJ, Varner JE (1987) Hydroxyproline-rich glycoprotein of plant cell walls. Trends Biol Sci 12: 24–27
Corbin DR, Sauer N, Lamb CJ (1987) Differential regulation of hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol 7: 4337–4344
Esquerré-Tugayé MT, Lamport DTA (1979) Cell surfaces in plantmicroorganism interactions. I: A structural investigation of cell wall hydroxyproline-rich glycoproteins which accumulate in fungus-infected plants. Plant Physiol 64: 314–319
Hahn MG, Bucheli P, Cervone F, Doares SH, O'Neill RA, Darvill A, Albersheim P (1989) The role of the cell wall constituents in plant-pathogen interactions In: Nester E, Kosuge T (eds) Plantmicrobe interactions, vol 3. McGraw-Hill, New York, pp 1–38
Harley JL (1989) The significance of mycorrhiza. Mycol Res 92: 92–129
Harlow E, Lane D (1988) Antibodies, a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Commonwealth Agricultural Bureaux Farnhan Royal, Bucks, pp 187–237
Knox JP (1990) Emerging patterns of organization at the plant cell surface. J Cell Sci 96: 557–561
Jarvis MC, Forsyth W, Duncan HJ (1988) A survey of the pectic content of nonlignified monocot cell walls. Annu Rev Biochem 53: 625–663
Lamport DTA (1965) The protein component of primary cell walls. Adv Bot Res 2: 151–218
— (1989) Extensin peroxidase ties the knots in the extensin network. In: Osborne DJ, Jackson MB (eds) Cell separation in plants. Springer, Berlin Heidelberg New York Tokyo, pp 101–113 (NATO ASI Series, series H, vol 35)
—, Miller DH (1971) Hydroxyproline arabinosides in the plant kingdom. Plant Physiol 48: 454–456
Ludevid MD, Ruiz-Avila L, Vallès MP, Stiefel V, Torrent M, Torné JM, Puigdomenech P (1990) Expression of genes for cell-wall proteins in dividing and wounded tissues ofZea mays L. Planta 180: 524–529
Mazau D, Esqueré-Tugayé MT (1986) Hydroxyproline-rich glycoprotein accumulation in the cell walls of plants infected by various pathogens. Physiol Mol Plant Pathol 29: 147–157
—, Rumeau D, Esquerré-Tugayé MT (1988) Two different families of hydroxyproline-rich glycoproteins in melon callus. Plant Physiol 86: 540–546
O'Connell RJ, Brown IR, Mansfield JW, Bailey JA, Mazau D, Rumeau D, Esquerré-Tugayé MT (1990) Immunocytochemical localization of hydroxyproline-rich glycoproteins accumulating in melon and bean at sites of resistance to bacteria and fungi. Mol Plant Microbe Interact 3: 33–40
Perotto S, VandenBosch KA, Brewin NJ, Faccio A, Knox JP, Bonfante-Fasolo P (1990) Modifications of the host cell wall during root colonization byRhizobium and VAM fungi. In: Nardon P, Gianinazzi-Pearson V, Grenier AM, Margulis M, Smith DC (eds) Endocytobiology IV. INRA Presse, Paris, pp 115–117
Showalter AM, Varner JE (1989) Plant hydroxyproline-rich glycoproteins. In: Abraham M (ed) The biochemistry of plants, vol 15, molecular biology. Academic Press, New York, pp 485–520
Spanu P, Boiler T, Ludwig A, Wiemken A, Faccio A, BonfanteFasolo P (1989) Chitinase in roots of mycorrhizalAllium porrum: regulation and localization. Planta 177: 447–455
Stafstrom JP, Staehelin LA (1988) Antibody localization of extensin in cell walls of carrot storage roots. Planta 174: 321–332
Stiefel V, Ruiz-Avila L, Raz R, Vallés MP, Gomez J, Pagés M, Martinez-Izquierdo JA, Ludevid MD, Langdale JA, Nelson T, Puigdoménech P (1990) Expression of a maize cell wall hydroxyproline-rich glycoprotein gene in early leaf and root vascular differentiation. Plant Cell 2: 785–793
van de Wiel C, Scheres B, Franssen H, Van Lierop MJ, Van Lammeren A, Van Kämmen A, Bisseling T (1990) The early nodulin transcript ENOD 2 is located in the nodule parenchyma (inner cortex) of pea and soybean root nodules. EMBO J 9: 1–7
Varner JE, Lin LS (1989) Plant cell wall architecture. Cell 56: 231–239
Wilson LG, Fry JC (1986) Extensin, a major cell wall glycoprotein. Plant Cell Environ 9: 239–260
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bonfante-Fasolo, P., Tamagnone, L., Peretto, R. et al. Immunocytochemical location of hydroxyproline rich glycoproteins at the interface between a mycorrhizal fungus and its host plants. Protoplasma 165, 127–138 (1991). https://doi.org/10.1007/BF01322283
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01322283