Summary
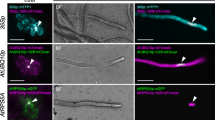
The technique of genetic cell ablation involves the targeted expression of a cell autonomous cytotoxic protein under the control of cell-specific regulatory sequences. This technique allows the investigation of cell-cell interactions by inducing selective death in a precisely controlled and cell autonomous manner. Here, targeted vegetative cell-specific ablation was used to examine the role of the vegetative cell (VC) in controlling generative cell (GC) behaviour and differentiation during pollen development. The tomatolat 52 late-pollen promoter, which has been shown to be activated specifically in the nascent VC immediately following pollen mitosis I (PMI), was used to direct expression of the cytotoxic diphtheria toxin A chain (DTA) in both transient expression assays using microprojectile bombardment and in transgenic tobacco plants. Transient expression of DTA linked to thelat 52 promoter (lot 52-DTA) in pollen dramatically reduced the expression of a co-transfected reporter gene fusion, demonstrating the cytotoxicity of DTA to pollen. Genetic and phenotypic analysis oflat 52-DTA transformants demonstrated that DTA expression led to a pollen-lethal phenotype, recognisable as small acytoplasmic pollen grains at anthesis, which affected 50% of the pollen population in single locus transformants. Detailed cytological analysis using confocal laser scanning microscopy and vital staining using fluorescein diacetate (FDA), showed that the first sign of cell ablation during pollen development was a loss of vital staining of the VC immediately following PMI. In contrast, the GC retained viability for up to several days following VC ablation, but progressively lost viability in the absence of a functional VC. Of particular interest was the observation that in the absence of VC function the generative cell (GC) failed to undergo normal migration away from the pollen grain wall into the VC cytoplasm. These results directly demonstrate the dependence of the GC on VC cell functions and highlight the importance of VC-GC interactions in controlling GC migration.
Similar content being viewed by others
Abbreviations
- CaMV:
-
cauliflower mosaic virus
- nos:
-
nopaline synthase
- DTA:
-
diptheria toxin A chain
- lat :
-
late anther tomato
- VC:
-
vegetative cell
- GC:
-
generative cell
- PGM:
-
pollen germination medium
- EtBr:
-
ethidium bromide
- FDA:
-
fluorescein diacetate
- FCR:
-
fluorochrome reaction
- DAPI:
-
4′,6-diamidino-2-phenylindole
References
An G, Czako M (1991) Expression of DNA coding for diphtheria toxin chain A is toxic to plant cells. Plant Physiol 95: 687–692
Bellen M, D'Evelyn D, Harvey M, Elledge SJ (1992) Isolation of temperature-sensitive diphtheria toxins in yeast and their effects onDrosophila cells. Development 114: 787–796
Breitman ML, Clapoff S, Rossant J, Tsui L-C, Glode M, Maxwell IH, Bernstein A (1987) Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science 238: 1563–1565
—, Rombola H, Maxwell IH, Klintworh GK, Bernstein A (1990) Genetic ablation in transgenic mice with an attenuated diphtheria toxin A gene. Mol Cell Biol 10: 474–479
Chapman GP (1987) The tapetum. Int Rev Cytol 107: 111–125
Czako M, Jyan-Chyun J, Herr JMJ, Marton L (1992) Differential manifestation of seed mortality induced by seed-specific expression of the gene for diphtheria toxin A chain inArabidopsis and tobacco. Mol Gen Genet 235: 33–40
Eady C, Lindsey K, Twell D (1994) Differential activation and conserved vegetative-cell-specific activity of a late pollen promoter in species with bi- and tricellular pollen. Plant J 5: 543–550
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol 45: 115–120
Höfgen R, Willmitzer L (1988) Storage of competent cells forAgrobacterium transformation. Nucleic Acids Res 16: 9877
Horsch R, Fry J, Hoffman N, Eichholtz D, Rogers S, Fraley R (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231
Kandasamy MK, Thorsness MK, Rundle SJ, Goldberg ML, Nasrallah JB, Nasrallah ME (1993) Ablation of papillar cell function inBrassica flowers results in the loss of stigma receptivity to pollination. Plant Cell 5: 263–275
Koltunow AM, Treuttner I, Cox KH, Wallroth M, Goldberg RB (1990) Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 2: 1201–1244
Koning A, Jones A, Fillatti JJ, Comai L, Lassner MW (1992) Arrest of embryo development inBrassica napus mediated by modifiedPseudomonas aeruginosa exotoxin A. Plant Mol Biol 18: 247–258
Mariani C, de Beuckeleer M, Truettner J, Leemans J, Goldberg RB (1990) Induction of male sterility in plants by a chimeric ribonuclease gene. Nature 347: 737–741
Maxwell F, Maxwell IH, Glode LM (1987) Cloning, sequence determination, and expression in transfected cells of the coding sequence for the tox 176 attenuated diphtheria toxin A chain. Mol Cell Biol 7: 1576–1579
Nasrallah JB, Nasrallah ME (1993) Pollen-stigma signalling in the sporophytic self-incompatibility response. Plant Cell 5: 1325–1335
Newbigin E, Anderson MA, Clarke AE (1993) Gametophytic self-incompatibility systems. Plant Cell 5: 1315–1324
Ow DW, Wood KV, DeLuca M, DeWet JR, Helinski DR, Howell SH (1986) Transient and stable expression of the firely lucife-rase gene in plant cells and transgenic plants. Science 234: 856–859
Pacini E (1990) Tapetum and microspore function. In: Blackmore S, Knox RB (eds) Microspores: evolution and ontogeny. Academic Press, London, pp 213–237
Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, Brinster RL (1987) Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell 50: 435–443
Pappenheimer AM (1977) Diphtheria toxin. Annu Rev Biochem 46: 69–94
Rogers SG, Klee HJ, Horsch RB, Fraley RT (1987) Improved vectors for plant transformation: expression cassette vectors and new selectable markers. Methods Enzymol 153: 253–277
Schlag M, Hesse M (1992) The formation of the generative cell inPolystachia pubescens (Orchidaceae). Sex Plant Reprod 5: 131–137
Schrauwen JAM, de Groot PFM, van Herpen MMA, van der Lee T, Reynen WH, Weterings KAP, Wullems GJ (1990) Stage-related expression of mRNAs during pollen development in lily and tobacco. Planta 182: 298–304
Thorsness MK, Kandasamy MK, Nasrallah ME, Nasrallah JB (1991) ABrassica S-locus gene promoter targets toxic gene expression and cell death to the pistil and pollen of transgenicNicotiana. Dev Biol 143: 173–184
— — — — (1993) Genetic ablation of floral cells inArabidopsis. Plant Cell 5: 253–261
Tupy J, Rihova L, Zarsky V (1991) Production of fertile tobacco pollen from microspores in suspension culture and its storage for in situ pollination. Sex Plant Reprod 4: 284–287
Twell D (1992) Use of a nuclear-targeted β-glucuronidase fusion protein to demonstrate vegetative cell-specific gene expression in developing pollen. Plant J 2: 887–892
— (1994) The diversity and regulation of gene expression in the pathway of male gametophyte development. In: Scott RJ, Stead AD (eds) Molecular and cellular aspects of plant reproduction. Cambridge University Press, Cambridge, pp 83–135 (Society for Experimental Biology, seminar series, vol 55)
—, Wing R, Yamaguchi J, McCormick S (1989 a) Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet 217: 240–245
—, Klein TM, Fromm ME, McCormick S (1989 b) Transient expression of chimeric genes delivered into pollen by microprojectile bombardment. Plant Physiol 91: 1270–1274
—, Yamaguchi J, McCormick S (1990) Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109: 705–713
— —, Wing RA, Ushiba J, McCormick S (1991) Promoter analysis of three genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes Dev 5: 496–507
Weterings KAP (1994) Pollen gene regulation. PhD Thesis, University of Nijmegen, Nijmegen, The Netherlands
—, Reijnen W, van Aarssen R, Kortstee A, Spijkers J, van Herpen M, Schrauwen J, Wullems G (1992) Characterization of a pollen-specific cDNA clone fromNicotiana tabacum expressed during microgametogenesis and germination. Plant Mol Biol 18: 1101–1111
Yamaizumi M, Mekada E, Uchida T, Okada Y (1978) One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 15: 245–250
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Twell, D. Diphtheria toxin-mediated cell ablation in developing pollen: Vegetative cell ablation blocks generative cell migration. Protoplasma 187, 144–154 (1995). https://doi.org/10.1007/BF01280243
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01280243