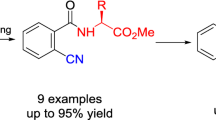
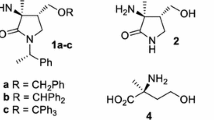
Summary
Strategies for the synthesis of optically active aspartaldehyde derivatives are reviewed. Most of them are using the chiral pool: allylglycine or naturally occurring homoserine, aspartic acid or methionme and side chain modifications. This will be developed in the first part. Some other original routes are also displayed in the second part. Different aspects of each strategy are discussed: the nature and number of steps, the problem of protecting groups, the price and availability of starting materials. Some synthetic applications of such interesting chiral synthons are shown in the last part.
Similar content being viewed by others
Abbreviations
- Ac:
-
acetyl
- An:
-
Anisyl or 4-methoxy benzyl
- Bn:
-
benzyl
- Boc:
-
tert-butoxycarbonyl
- BOP-PF6 :
-
benzotriazol-l-yloxytris(dimethylamino)phosphonium hexafluorophosphate
- Cbz:
-
benzyloxycarbonyl
- DCC:
-
dicyclohexylcarbodiimide
- DIBAL:
-
diisobutyl aluminum hydride
- DIPEA:
-
diisopropyl ethyl amine
- DMF:
-
dimethyl formamide
- DMSO:
-
dimethylsulfoxide
- EDCI:
-
l-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride
- HP:
-
4-hydroxy phenyl
- MP:
-
4-methoxy phenyl
- NCS:
-
N-chlorosuccinimide
- NMR:
-
nuclear magnetic resonance
- PCC:
-
pyridinium chlorochromate
- Pht:
-
phthaloyl
- Ser:
-
serine
- tBu:
-
tert-butyl
- TEMPO:
-
2,2,6,6-tetramethyl piperidine-l-oxyl
- TFA:
-
trifluoro acetic acid
- Trityl:
-
triphenyl methyl
- Val:
-
valine
References
Agami C, Couty F, Poursoulis M (1992) Aza-Cope rearrangement. Asymmetric syntheses ofα-amino acids: (R)-homoserine lactone and (R,R)-4-(l-hydroxy-l-methylethyl)proline. Synlett: 847–848
Agami C, Couty F, Lin J, Mikaeloff A, Pousoulis M (1993) Asymmetric synthesis ofα-amino acids via cationic aza-Cope rearrangements. Tetrahedron 49: 7239–7250
Albertson NF (1946) The synthesis of amino acids from ethyl acetamidomalonate and ethyl acetamidocyanoacetate. III. The use of primary halides. J Am Chem Soc 68: 450–453
Baldwin JE, Flinn A (1987) Use of L-aspartic acidβ-semialdehyde in the synthesis of more compex non protein amino acids. Tetrahedron Lett 28: 3605–3608
Baldwin JE, Lee E (1986) Synthesis of bicyclicγ-lactams via oxazolidmones. Tetrahedron 42: 6551–6554
Baldwin JE, Lowe C, Schofield CJ, Lee E (1986) Aγ-lactam analogue of penems possessing antibacterial activity. Tetrahedron Lett 27: 3461–3464 and 5042
Baldwin JE, North M, Flinn A (1987) A convenient new synthesis of homoserine lactone. Tetrahedron Lett 28: 3167–3168
Baldwin JE, Norris WJ, Freeman RT, Bradley M, Adlington RM, Long-Fos S, Schofield CJ (1988a)γ-Lactam formation from tripeptides with isopenicillin N synthase. J Chem Soc Chem Com: 1128–1130
Baldwin JE, North M, Flinn A (1988b) Synthesis and rearrangement of homoserine derivatives. Tetrahedron 44: 637–642
Baldwin JE, Freeman RT, Schofield C (1989a) Synthesis of a novel bicyclicγ-lactam analogue of the 1-oxapenams. Tetrahedron Lett 30: 4019–4020
Baldwin JE, Freeman RT, Lowe C, Schofield CJ, Lee E (1989b) Aγ-lactam analogue of the penems possessing antibacterial activity. Tetrahedron 45: 4537–4550
Barrett GC (1985) Chemistry and biochemistry of the amino acids. In: Barrett GC (ed) Chapman and Hall, London New York, pp 246–296
Ben-Bari M, Dewynter G, Aymard C, Jei T, Montero J-L (1995) Synthèse de dérivés phosphonylés de l'acide aspartique inhibiteurs potentiels de l'ATCase. Phosphorus, Sulfur and Silicon 105: 129–144
Bergmeier SC, Cobas AA, Rapoport H (1993) Chirospecific synthesis of (1S, 3R)-l-amino-3-(hydroxymethyl)cyclopentane, precursor for carbocyclic nucleoside synthesis. Dieckmann cyclisation with anα-amino acid. J Org Chem 58: 2369–2376
Black S, Wright NG (1955a)β-Aspartokinase andβ-aspartyl phosphate. J Biol Chem 213: 27–38
Black S, Wright NG (1955b) Asparticβ-semialdehyde dehydrogenase and asparticβ-semialdehyde. J Biol Chem 213: 39–50
Black S, Wright NG (1955c) Homoserine dehydrogenase. J Biol Chem 213: 51–60
Blickling S, Renner C, Laber B, PohlenzH-D, Holak TA, Huber R (1997) Reaction mechanism ofEscherichia coli dihydrodipicolinate synthase investigated by X-ray crystallography and NMR spectroscopy. Biochemistry 36: 24–33
Bold G, Steiner H, Moesch L, Walliser B (1990) Herstellung von “Semialdehyd”-Derivaten von Asparaginsäure-und Glutaminsäure durch Rosenmund-Reduktion. Helv Chim Acta 73: 405–410
Bratusek U, Kejzar I, Svete J, Stanovnik B (1996) The synthesis of N-phthaloyl-azatryptophane derivatives. Acta Chim Slov 43: 105–117
Bubert C, Voigt J, Biasetton S, Reiser O (1994) A new approach toβ- andγ amino esters and amino aldehydes by regioselective ozonolysis of 2,3-dihydropyrroles and 1,2,3,4-tetrahydropyridines. Synlett: 675–677
Burger K, Rudolph M, Fehn S, Worku A, Gobulev A (1995) Application of hexafluoroacetone as protecting and activating reagent in amino acid and peptide chemistry. Amino Acids 8: 195–199
Cannarsa MJ (1996) Single enantiomer drugs: new strategies and directions. Chem Ind: 374–378
Cavrini V, Chiarini A, Garuti L, Giovanninetti G, Franchi L (1976) Riceche su sostanze ad attivita antivirale. Nota V - analoghi strutturali dell'arginina. Farmaco Ed Sci 31: 599–606
Chang MNT, Walsh CT (1981) Stereochemical analysis ofγ-replacement andγ-elimination processes catalyzed by a pyridoxal phosphate dependent enzyme. J Am Chem Soc 103: 4921–4927
Chauvel EN, Llorens-Cortes C, Coric P, Wilk S, Roques BP, Fournié-Zaluski M-C (1994) Differential inhibition of aminopeptidase A and aminopeptidase N by newβ-amino thiols. J Med Chem 37: 2950–2957
Cooper RDG, Jose F, McShane L, Koppel GA (1978) A chiral synthesis of D-homoserine and its application to the synthesis of nocardicin A. Tetrahedron Lett: 2243–2246
Coppola GM (1987) Asymmetric synthesis: construction of chiral molecules using amino acids. John Wiley and Sons, New York
Coulter CV, Gerrard JA, Kraunsoe JAE, Moore DJ, Pratt AJ (1996) (S)-Aspartate semialdehyde: synthetic and stuctural studies. Tetrahedron 52: 7127–7136
Cox RJ, Sherwin WA, Lam LKP, Vederas JC (1996) Synthesis and evaluation of novel substrates and inhibitors of N-succinyl-L,L-diaminopimelate aminotransferase (DAPAT) fromEscherichia coli. J Am Chem Soc 118: 7449–7460
Crosby J (1992) Chirality in industry: the commercial manufacture and applications of optically active compounds, vol 1. In: Collins AN, Sheldrake GN, Crosby J (eds) John Wiley and Sons, Chichester, pp 1–66
Crosby J (1997) Chirality in industry II: developments in the manufacture and applications of optically active compounds, vol 2. In: Collins AN, Sheldrake GN, Crosby J (eds) John Wiley and Sons, Chichester, pp 1–10
Cuvinot D, Mangeney P, Alexakis A, Normant JF, Lellouche JP (1989) Chiral trifluoro diamines as convenient reagents for determining the enantiomeric purity of aldehydes by use of19F NMR spectroscopy. J Org Chem 54: 2420–2425
David S, Veyrieres A (1970) Synthesis of 3-amino-2,3-dideoxytetrose derivatives. Carbohyd Res 13: 203–209
Davies IW, Reider PJ (1996) Practical asymmetric synthesis. Chem Ind: 412–415
De la Figuera N, Rozas I, Garcia-Lopez MT, Gonzales-Muniz R (1994) 2(S)-Amino-3-oxo-11b(R)-hexahydroindolizino[8,7-b]indole-5(S)-carboxylate as a new type ofβ-turn dipeptide mimetic. J Chem Soc Chem Commun: 613–614
De la Figuera N, Alkorta I, Garcia-Lopez MT, Herranz R, Gonzales-Muniz R (1995) 2-Amino-3-oxohexahydroindolizino[8,7-b]indole-5-carboxylate derivatives as new scaffolds for mimickingβ-turn secondary structures. Molecular dynamics and stereoselective synthesis. Tetrahedron 51: 7841–7856
Dehmlow EV, Westerheide R (1993) A new simple route to N,O-protected (S)-2-amino-4-oxobutyric acid. Synthesis: 1225–1226
Duthaler RO (1994) Recent developments in the stereoselective synthesis ofα-amino acids. Tetrahedron 50: 1539–1650
Easton CJ, Hutton CA (1998) Recent developments in the use of N-phthaloyl-amino acid derivatives in synthesis. Synlett: 457–466
Emmer G, Grassberger MA, Schulz G, Boesch D, Gavériaux C, Loor F (1994) Derivatives of a novel cyclopeptolide. 2. Synthesis, activity against multidrug resistance in CHO and KB cellsin vitro, and structure-activity relationships. J Med Chem 37: 1918–1928
Faust J, Schreiber K (1989) Der Normal isierungsfaktor für die Tomatenmutante Chloronerva; Synthese des (S)-Piperidin-2-Carbonsäureanalogons von Nicotianamin. Z Chem 29: 20–21
Faust J, Preiss A, Shreiber K, Ripperger H (1983) On the “normalizing” factor for the tomato mutant Choronerva-XIV: synthesis of the proline analogue of the phytosiderophore nicotianamine. Tetrahedron 39: 1593–1596
Faust J, Schreiber K, Ripperger H (1984) Der “Normalisierungsfaktor” für die Tomatenmutante Chloronerva: ein einfacher Zugang zum “Aldehydbaustein” der Nicotianaminesynthese. Z Chem 24: 330–331
Fushiya S, Nakatsuyama S, Sato Y, Nozoe S (1981a) Synthesis of nicotianamine and related compound, derivatives of azetidine-2-carboxylic acid. Heterocycle 15: 819–822
Fushiya S, Sato Y, Nakatsuyama S, Kanuma N, Nozoe S (1981b) Synthesis of avenic acid A and 2′-deoxymugineic acid, amino acids possessing an iron chelating activity. Chem Lett: 909–912
Fushiya S, Maeda K, Funayama T, Nozoe S (1988a) 4-N-Hydroxy-L-2,4-diaminobutyric acid. A strong inhibitor of glutamine synthetase. J Med Chem 31: 480–483
Fushiya S, Watari F, Tashiro T, Kuzano G, Nozoe S (1988b) A new acidic amino acid from a basidiomycetes, lactarius piperatus. Chem Pharm Bull 36: 1366–1370
Gao Y, Lane-Bell P, Vederas JC (1998) Stereoselective synthesis of meso-2,6-diaminopimelic acid and its selectively protected derivatives. J Org Chem 63: 2133–2143
Garvey DS, May PD, Nadzan AM (1990) 3,4-Disubstitutedγ-lactam rings as conformationally constrained mimics of peptide derivatives containing aspartic acid or norleucine. J Org Chem 55: 936–940
Genêt J-P, Thorimbert S, Touzin A-M (1993) Palladium(0) catalyzed amination with N,O-bis-ter-Boc hydroxylamine. Synthesis of (+)-N6-hydroxylysine. Tetrahedron Lett 34: 1159–1162
Geze M, Blanchard P, Fourrey JL, Robert-Gero M (1983) Synthesis of sinefungin and its C-6′ epimer. J Am Chem Soc 105: 7638–7640
Giorgianni F, Beranova S, Wesdemiotis C, Viola RE (1995) Elimination of the sensitivity of L-aspartase to active-site-directed inactivation without alteration of catalytic activity. Biochemistry 34: 3529–3535
Girard A, Greek C, Genêt JP (1998) Rapid syntheses of 3-amino-5-hydroxymethyl-γ-lactones from L-allylglycine. Tetrahedron Lett 39: 4259–4260
Hanessian S, Yang R-J (1996) The asymmetric synthesis of allylglycine and other unnaturalα-amino acids via zinc-mediated allylation of oximes in aqueous media. Tetrahedron Lett 37: 5273–5276
Hoffmann MG, Zeiss H-J (1992) A novel and convenient route to L-homoserine lactones and L-phosphinothricin from L-aspartic acid. Tetrahedron Lett 33: 2669–2672
Itokawa H, Kondo K, Hitotsuyanagi Y, Isomura M, Takeya K (1993) Studies on RA derivatives. V. Synthesis and antitumor activity of Ala2-modified RA-VII derivatives. Chem Pharm Bull 41: 1402–1410
Jungheim LN, Boyd DB, Indelicato JM, Pasini CE, Preston D, Alborn WE (1991) Synthesis, hydrolysis rates, supercomputer modeling, and antibacterial activity of bicyclic tetrahydropyridazinones. J Med Chem 34: 1732–1739
Katagiri N, Okada M, Kaneko C, Furuya T (1996) Synthesis of chiral cyclic nitrones via a nitrosoketene intermediate and their use for the complete EPC synthesis of nonproteinogenic amino acids. Tetrahedron Lett 37: 1801–1804
Kazmaier U (1996) Synthesis ofγ,δ-unsaturated amino acids via ester enolate Claisen rearrangement of chelated allylic esters. Amino Acids 11: 283–299
Keith DD, Tortora JA, Ineichen K, Leimgruber W (1975) The total synthesis of rhizobitoxine. Tetrahedron 31: 2633–2636
Keith DD, Tortora JA, Yang R (1978) Synthesis of L-2-amino-4-methoxy-trans-but-3-enoic acid. J Org Chem 43: 3711–3713
Knapp S, Hale JJ, Bastos M, Molina A, Chen KY (1992) Synthesis of hypusine and other polyamines using dibenzyltriazones for amino protection. J Org Chem 57: 6239–6256
Koch T, Buchardt O (1993) Synthesis of L-(+)-selenomethionine. Synthesis: 1065–1067
Kokotos G, Padron JM, Martin T, Gibbons WA, Martin VS (1998) A general approach to the asymmetric synthesis of unsaturated lipidic (α-amino acids. The first synthesis ofα-amino arachidonic acid. J Org Chem (in press)
Matsuura F, Hamada Y, Shioiri T (1994a) Total syntheses of phytosiderophores, 3-epihydroxymugineic acid, distichonic acid A, and 2′-hydroxynicotianamine. Tetrahedron 50:265–274
Matsuura F, Hamada Y, Shioiri T (1994b) Total synthesis of 2′-deoxymugineic acid and nicotianamine. Tetrahedron 50: 9457–9470
Meffre (1998) In different reaction conditions, this strategy may lead to the formation of vinylsulfide in good yield. Synthesis (submitted to publication)
Meffre P, Lhermitte H, Vo-Quang L, Vo-Quang Y, Le Goffic F (1991) A new class of unusualα-amino acids:α-amino acidβ,γ-enol thioethers. Tetrahedron Lett 32: 4717–4720
Meffre P, Durand P, Le Goffic F (1995) Straightforward synthesis of optically pure methyl (S)-2-phthalimido-4-oxobutanoate. Synthesis: 1111–1114
Meffre P, Durand P, Le Goffic F (1999) Methyl (S)-2-phthalimido-4-oxobutanoate. Org Synth 76 (in press)
Metzler DE (1977) Biochemistry: the chemical reactions of living cells. Academic Press Inc, New York, p 824
Mock GA, Moffatt JG (1982) An approach to the total synthesis of sinefungin. Nucleic Acid Res 10: 6223–6234
Myers AG, Gleason JL, Yoon T, Kung DW (1997) Highly practical methodology for the synthesis of D- and L-α-amino acids, N-protectedα-amino acids, and N-methyl-α-amino acids. J Am Chem Soc 119: 656–673
Neuberger A, Tait GH (1962) Synthesis of L-asparticβ-semialdehyde. J Chem Soc: 3963–3968
Ohfune Y, Nomoto K (1981) Synthesis of (+)-avenic acid A. Chem Lett: 827–828
Ohfune Y, Tomita M, Nomoto K (1981) Total synthesis of 2′-deoxyugineic acid, the metal chelator excreted from wheat root. J Am Chem Soc 103: 2409–2410
Oida F, Ota N, Mino Y, Nomoto K, Sugiura Y (1989) Stereospecific iron uptake mediated by phytosiderophore in gramineous plants. J Am Chem Soc 111: 3436–3437
Ornstein PL, Melikian A, Martinelli MJ (1994) Intramolecular Diels - Alder route to 6-oxodecahydroisoquinoline-3-carboxylates: intermediates for the synthesis of conform ationally constrained excitatory amino acid antagonists. Tetrahedron Lett 35: 5759–5762
Pellegrini M, Weitz IS, Chorev M, Mierke DF (1997) A trisubstituted 1,4-diazepine-3-one-based dipeptidomimetic: conformational characterization by NMR and computer simulation. J Am Chem Soc 119: 2430–2436
Perish JW (1992) The facile synthesis of 2-(fluorenylmethoxycarbonylamino)-4-(O′, O″-dim ethyl phosphono) -L-butanoic acid {Fmoc-Abu(PO3Me2)-OH}. Synlett: 595–596
Perish JW (1994) Efficient Fmoc/solid-phase synthesis of Abu(P)-containing peptides using Fmoc-Abu(PO3Me2)-OH. Int J Peptide Protein Res 44: 288–294
Ramalingam K, Woodward RW (1988) Synthesis of stereospecific deuterium-labeled homoserines and homoserine lactones. J Org Chem 53: 1900–1903
Ramsamy K, Olsen RK, Emery T (1982) Synthesis of N-t-Boc-L-α-aminoadipic acid l-t-butyl 6-ethyl ester from L-aspartic acid: a new route to L-α-aminoadipic acid. Synthesis: 42–43
Richards A, McCague R (1997) The impact of chiral technology on the pharmaceutical industry. Chem Ind: 422–425
Ripperger H (1988) Synthesis of 3,4-seconicotianamine. J Prakt Chem 330: 470–472
Robl JA, Karanewsky DS, Asaad MM (1995) Synthesis of benzo-fused, 7,5- and 7,6-fused azepinones as conformationally restricted dipeptide mimetics. Tetrahedron Lett 36: 1593–1596
Schindler JF, Viola RE (1994) Mechanism-based inactivation of L-aspartase fromEscherichia coli. Biochemistry 33: 9365–9370
Shioiri T, Irako N, Sakakibari S, Matsuura F, Hamada Y (1997) A new efficient synthesis of nicotianamine and 2′-deoxymugineic acid. Heterocycles 44: 519–530
Shono T, Matsumura Y, Tsubata K, Sugihara Y, Yamane S-I, Kanazawa T, Aoki T (1982) Electroorganic chemistry. 60. Electroorganic synthesis of enamides and enecarbamates and their utilization in organic synthesis. J Am Chem Soc 104: 6697–6703
Stockman RA, Szeto P, Thompson SHJ, Hadley MS, Lathbury DC, Gallagher T (1996) Synthesis of functionalised azabicycles via a regiospecific intramolecular aldol reaction. Synlett: 853–855
Subasinghe NL, Bontems RJ, McIntee E, Mishra RK, Johnson RL (1993) Bicyclic thiazolidine lactam peptidomimetics of the dopamine receptor modulating peptide Pro-Leu-Gly-NH,. J Med Chem 36: 2356–2361
Svete J, Stanovnic B, Tisler M (1994) The synthesis of azatryptophane derivatives. J Heterocyclic Chem 31: 1259–1266
Szeto P, Lathbury DC, Gallagher T (1995) Heterocyclic ketones as pre-formed building blocks. Synthesis of (-)-slaframine. Tetrahedron Lett 36: 6957–6960
Tong G, Perich JW, Johns RB (1990) Synthesis of Leu-Abu(P)and Glu-Abu(P)-Leu. Isosteres of Ser(P)-Peptides. Tetrahedron Lett 31: 3759–3762
Tong G, Perich JW, Johns RB (1992) The improved synthesis of Boc-Abu(PO3Me2)-OH and its use for the facile synthesis of Glu-Abu(P)-Leu. Aust J Chem 45: 1225–1240
Toujas JL, Jost E, Vaultier M (1997) Synthesis of homochiral N-Boc-β-aminoaldehydes from N-Boc-β-aminonitriles. Bull Soc Chim Fr 134: 713–717
Townsend CA, Reeve AM, Salituro GM (1988) Stereochemical fate of (2S, 4R)- and (2S, 4S)-[4-2H] methionine in norcardicin A biosynthesis. J Chem Soc Chem Com: 1579–1581
Tudor DW, Lewis T, Robins DJ (1993) Synthesis of the trifluoroacetate salt of aspartic acidβ-semialdehyde, an intermediate in the biosynthesis of L-lysine, L-threonine, and L-methionine. Synthesis: 1061–1062
Uzar HC (1991) Improved synthesis of L-homoserine derivatives from L-aspartic acid. Synthesis: 526–528
Valerio RM, Alewood PF, Johns RB (1988) Synthesis of optically active 2-tert-butyloxycarbonylamino)-4-dialkoxyphosphorylbutanoate protected isosteres of o-phosphoserine for peptide synthesis. Synthesis: 786–789
Venkatraman S, Roon RJ, Schulte MK, Koerner JF, Johnson RL (1994) Synthesis of oxadiazolidinedione derivatives as quisqualic acid analogues and their evaluation at a quisqualate-sensitized site in the rat hippocampus. J Med Chem 37: 3939–3946
Walsh C (1982) Suicide substrate: mechanism-based enzyme inactivators. Tetrahedron 38: 871–909
Weinkam RJ, Jorgensen EC (1971) Free radical analogs of histidine. J Am Chem Soc 93: 7028–7033
Weitz IS, Pellegrini M, Mierke DF, Chorev M (1997) Synthesis of a trisubstituted 1,4diazepine-3-one-based dipeptidomimetic as a novel molecular scaffold. J Org Chem 62: 2527–2534
Werner RM, Shokek O, Davis JT (1997) Preparation of 4-oxo-L-norvaline via diazomethane homologation ofβ-aspartyl semialdehyde. J Org Chem 62: 8243–8246
Wernic D, DiMaio J, Adams J (1989) Enantiospecific synthesis of L-α-aminosuberic acid. Synthetic applications in preparation of atrial natriuretic factor analogues. J Org Chem 54: 4224–4228
Weygand F, Fritz H (1965) Über N-TFA-Asparaginsäure-Derivate. Chem Ber 98: 72–82
Williams RM (1989) Synthesis of optically activeα-amino acids. Pergamon Press, Oxford
Williams RM, Liu J (1998) Asymmetric synthesis of differentially protected 2,7-diaminosuberic acid, a ring closure methatesis approach. J Org Chem 63: 2130–2132
Winkler D, Burger K (1996) Synthesis of enantiomerically pure D- and L-Armentomycin and its difluoro analogues from aspartic acid. Synthesis: 1419–1421
Author information
Authors and Affiliations
Additional information
This paper is dedicated to RV.
Rights and permissions
About this article
Cite this article
Meffre, P.R. Syntheses of optically active 2-amino-4-oxobutyric acid and N,O-protected derivatives. Amino Acids 16, 251–272 (1999). https://doi.org/10.1007/BF01388171
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01388171