Abstract
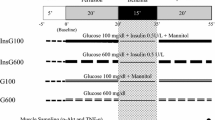
Depletion of gycogen has been proposed as the mechanism of protection from ischemic preconditioning. The hypothesis was tested by seeing whether pharmacological manipublation of preconditioning causes parallel changes in cardiac glycogen content. Five groups of isolated rabbit hearts were studied. Group 1 experienced 30 min of ischemia only. Group 2 (PC) was preconditioned with 5 min of global ischemia followed by 10 min of reperfusion. Group 3 was preconditioned with 5 min exposure to 400 nM bradykinin followed by a 10 min washout period. Group 4 experienced exposure to 10 μM adenosine followed by a 10 min washout period, and the fifth group was also preconditioned with 5 min ischemia and 10 min reperfusion but 100 μM8-(p-sulfophenyl) theophylline (SPT), which blocks adenosine receptors, was included in the buffer to block preconditioning's protection. Transmural biopsies were taken before treatment, just prior to the 30 min period of global ischemia, and after 30 min of global ischemia. Glycogen in the samples was digested with amyloglucosidase and the resulting glucose was assayed. Baseline glycogen averaged 17.3±0.6 μmol glucose/g wet weight. After preconditioning glycogen decreased to 13.3±1.3 μmol glucose/g wet weight (p<0.005 vs. baseline). Glycogen was similarly depleted after pharmacological preconditioning with adenosine (14.0±1.0 μmol glucose/g wet weight, p<0.05 vs. baseline) suggesting a correlation. However, when proconditioning was performed in the pressence of SPT, which blocks protection, glycogen was also depleted by the same amount (13.3±0.7 μmol glucose/g wet weight, p=ns vs. PC). Bradykinin, which also mimics preconditioning, caused no depletion of glycogen (16.3±0.8 μmol glucoseig wet weight, p=ns vs. baseline). Because preconditioning with bradykinin did not deplete glycogen and because glycogen continued to be low when protection from preconditioning was blocked with SPT, we conclude that loss of glycogen per se does not cause the protection of preconditioning.
Similar content being viewed by others
References
de Albuquerque CP, Gerstenblith G, Weiss RG (1994). Importance of metabolic inhibition and cellular pH in mediating preconditioning contractile and metabolic effects in rat hearts. Circ Res. 74:139–150
Asimakis GK (1996) Myocardial glycogen depletion cannot explain the cardioprotective effects of ischemic preconditioning in the rat heart. J Mol Cell Cardiol 28:563–570
Bøtker HE, Kimose HH, Thomassen AR, Nielsen TT (1995) Applicability of small endomyocardial biopsies for evaluation of high energy phosphates and glycogen in the heart. J Mol Cell Cardiol 27:2081–2089
Bøtker HE, Randsbæk F, Hansen SB Thomassen A, Nielsen TT (1995) Superiority of acid extractable glycogen for detection of metabolic changes during myocardial ischaemia. J Mol Cell Cardiol 27:1325–1332
Brew EC, Mitchell MB, Rehring TF, Gamboni-Robertson F, McIntyre RC, Harken AH, Banerjee A (1995) Role of bradykinin in cardiac functional protection after global ischemia-reperfusion in rat heart. Am J Physiol 269:H1370-H1378
Buxton DB, Fisher RA, Robertson SM, Olson MS (1987) Stimulation of glycogenolysis and vasoconstriction by adenosine and adenosine analogues in the perfused rat liver. Biochem J 248:35–41
Buxton DB, Robertson SM, Olson MS (1986) Stimulation of glycogenolysis by adenine nucleotides in the perfused rat liver. Biochem J 237:773–780
Carr RS, Neff JM (1984) Quantitative semi-automated enzymatic assay for tissue glycogen. Comp. Biochem Physiol 77B:447–449
Cross HR, Opie LH, Radda GK, Clarke K (1996) Is a high glycogen content beneficial or detrimental to the ischemic rat heart? A controversy resolved. Circ Res 78:482–491
Finegan BA, Lopaschuk GD, Coulson CS, Clanachan AS (1993) Adenosine alters glucose use during ischemia and reperfusion in isolated rat hearts. Circulation 87:900–908
Finegan BA, Lopaschuk GD, Gandhi M, Clanachan AS (1995) Ischemic preconditioning inhibits glycolysis and proton production in isolated working rat hearts. Am J Physiol 269:H1767-H1775
Goodwin GW, Taegtmeyer H (1994) Metabolic recovery of isolated working rat heart after brief global ischemia. Am J Physiol 267:H462-H470
Goto M, Cohen MV, Van Wylen DGL, Downey JM (1996) Attenuated purine production during subsequent ischemia in preconditioned rabbit myocardium is unrelated to the mechanism of protection. J Mol Cell Cardiol 28:447–454
Goto M, Liu Y, Yang XM, Ardell JL Cohen MV, Downey JM (1995) Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res 77:611–621
Ikonomidis JS, Shirai T, Weisel RD, Derylo B, Rao, V, Whiteside CI, Mickle DAG, Li R-K (1995) “Ischemic” or adenosine preconditioning of human ventricular cardiomyocytes is protein kinase C dependent. Circulation 92:I-12
Jennings RB, Reimer KA, Steenbergen C, Schaper J (1989) Total ischemia III: Effect of inhibition of anaerobic glycolysis. J Mol Cell Cardiol 21 Suppl 1:37–54
Lagerstrom CF, Walker WE, Taegtmeyer H (1988) Failure of glycogen depletion to improve, left ventricular function of the rabbit heart after hypothermic ischemic arrest. Circ Res 63:81–86
Lawson CS, Downey JM (1993) Preconditioning: state of the art myocardial protection. Cardiovasc Res 27:542–550
Liu GS, Richards SC, Olsson RA, Mullane K, Walsh RS, Downey JM (1994) Evidence that the adenosine A3 receptor may mediate the protection afforded by preconditioning in the isolated rabbit heart. Cardiovasc Res 28:1057–1061
Liu GS, Thornton JD, Van Winkle DM, Stanley AW, Olsson RA, Downey JM, (1991) Protection against infarction afforded by preconditioning, is mediated by A1 adenosine receptors in rabbit heart. Circulation 84:350–356
Liu Y, Downey JM (1992) Ischemic preconditioning protects against infarction in rat heart. Am J Physiol 263:H1107-H1112
Miura T, Iimura O (1993) Infarct size limitation by preconditioning: its phenomenological features and the key role or adenosine. Cardiovasc Res 27:36–42
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136
Murry CE, Richard VJ, Reimer KA, Jennings RB (1990) Ischemic preconditioning slow energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res 66:913–931
Neely JR, Grotyohann LW (1984) Role of glycolytic products in damage to ischemic myocardium. Dissociation of adenosine triphosphate levels and recovery of function of reperfused ischemic hearts. Circ Res 55:816–824
Nukina S, Fusaoka T, Thurman RG (1994) Glycogenolytic effect of adenosine involves ATP from hepatocytes and eicosanoids from Kupffer cells. Am J Physiol 266:G99–105
Parratt JR, Vegh A, Papp JG (1995) Bradykinin as an endogenous myocardial protective substance with particular reference to ischemic preconditioning—a brief review of the evidence. Can J Physiol Pharmacol 73:837–842
Reimer KA, Jennings RB (1986) Myocardial ischemia, hypoxial ano infarction. In: Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE (eds) The heart and cardiovascular system. Raven Press, New York 1133–1201
Schaefer S, Carr LJ Prussel E, Ramasamy R (1995) Effects of glycogen depletion on ischemic injury, in isolated rat hearts: insights into preconditioning. Am J Physiol 268:H935-H944
Shizukuda Y, Iwamoto T, Mallet RT, Downey HF (1993) Hypoxic preconditioning attenuates stunning caused by repeated coronary artery occlusion in dog hearts. Cardiovasc Res 27:559–564
Tsuchida A, Liu Y, Liu GS, Cohen MV, Downey JM (1994) α1-Adrenergic agonists precondition rabbit ischemic myocardium independent of adenosine by direct activation of protein kinase C. Circ Res 75:576–585
Vander Heide RS, Reimer KA, Jennings RB (1993) Adenosine slows ischaemic metabolism in canine myocardium in vitro: relationship to ischaemic preconditioning. Cardiovasc Res 27:669–673
Vanoverschelde J-LJ, Janier MF, Bakke JE, Marshall DR, Bergmann SR (1994) Rate of glycolysis during ischemia determines extent of ischemic injury and functional recovery after reperfusion. Am J Physiol 267:H1785-H1794
Vanstapel F, Waebens M, Van Hecke P, Decanniere C, Stalmans W (1991) Modulation of maximal glycogenolysis in perfused rat liver by adenosine and ATP. Biochem J 277:597–602
Volovsek A, Subramanian R, Reboussin D (1992) Effects of duration of ischaemia during preconditioning on mechanical function, enzyme release and energy production in the isolated working rat heart. J Mol Cell Cardiol 24:1011–1019
Walsh RS, Borges M, Thornton JD, Cohen MV, Downey JM (1995) Hypoxia preconditions rabbit myocardium by an adenosine receptor-mediated mechanism. Can J Cardiol 11:141–146
Wolfe CL, Sievers RE, Visseren FLJ, Donnelly TJ (1993) Loss of myocardial protection after preconditioning correlates with the time course of glycogen recovery within the preconditioned segment. Circulation, 87:881–892
Woolfson RG, Patel VC, Yellon DM (1996) Pre-conditioning with adenosine leads to concentration-dependent infarct size reduction in the isolated rabbit heart. Cardiovasc Res 31:148–151
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weinbrenner, C., Wang, P. & Downey, J.M. Loss of glycogen during preconditioning is not a prerequisite for protection of the rabbit heart. Basic Res Cardiol 91, 374–381 (1996). https://doi.org/10.1007/BF00788717
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00788717