Abstract
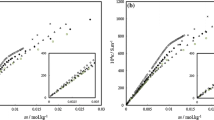
Thermodynamics of surfactant-dye complex formation have been studied, in terms of equilibrium coefficient, using a spectrophotometer. The systems are 6 sodium alkyl sulfates, which have different alkyl chain lengths, and 4-phenylazo-1-naphthylamine. A pronounced spectral change in the dye solution occurs on addition of the surfactant; the change has a definite isosbestic point and a new absorption band at 535 nm because of surfactant-dye complex formation, which is caused by hydrophilic-hydrophilic interaction. As the alkyl chain length in the surfactant increases, the values of free energy change (negative) increase, while the value of enthalpy change (negative) increases and the value of entropy change (positive) decreases. The longer the alkyl chain length in surfactant increase, the more stable the surfactant-dye complex becomes.
Surfactant-dye complex will form due to hydrophilic-hydrophilic interaction and will become more stable due to hydrophobic-hydrophobic interaction.
Similar content being viewed by others
References
Nemoto Y, Funahashi H (1977) J Coll Interf Sci 62:95
Nemoto Y, Funahashi H (1974) Yukagaku 23:75
Luck W (1958) J Soc Dyers Colourists 74:221
Craven BR, Datyner A (1961) J Soc Dyers Colourists 77:304
Hiskey CF, Downey TA (1954) J Phys Chem 58:835
Abe M, Ohsato M, Kawamura T, Ogino K (1985) J Coll Interf Sci 104:228
Abe M, Ohsato M, Uchiyama H, Tsubaki N, Ogino K (1986) J Jpn Oil Chem Soc 35:522
Uchiyama H, Abe M, Ogino K (1986) J Jpn Oil Chem Soc 35:1031
Abe M, Suzuki N, Ogino K (1983) J Coll Interf Sci 93:285
Abe M, Suzuki N, Ogino K (1984) J Coll Interf Sci 99:226
Abe M, Ohsato M, Suzuki N, Ogino K (1984) Bull Chem Soc Jpn 57:831
Abe M, Ohsato M, Ogino K (1984) Coll Polym Sci 262:657
Abe M, Kasuya T, Ogino K (1986) J Jpn Oil Chem Soc 35:718
Abe M, Ohsato M, Kasuya T, Ogino K (1986) J Jpn Oil Chem Soc 35:958
Tanizaki Y (1966) Bull Chem Soc Jpn 39:718
Kuroiwa S, Ogasawara S (1968) Sen'i Gakkai Shi 24:536
Mitsuishi M (1964) Sen'i Gakkai Shi 20:657
Nemethy G, Scherage HA (1962) J Phys Chem 66:1773
Nakamura A, Muramatsu M (1977) J Coll Interf Sci 62:165
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abe, M., Kasuya, T. & Ogino, K. Thermodynamics of surfactant-dye complex formation in aqueous solutions. Sodium alkyl sulfates and azo oil dye systems. Colloid & Polymer Sci 266, 156–163 (1988). https://doi.org/10.1007/BF01452813
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01452813