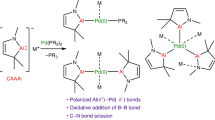
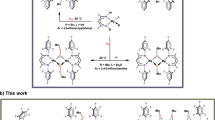
Abstract
THE manganese pentacarbonyl halides react with a variety of ligands of the type PR3 and P(OR)3 to give mono- and di-substituted products, Mn(CO)4LX and Mn(CO)3L2X 1,2. The reaction of 0.2 g Mn(CO)5Br with 1.1 g P(OC6H5)3 in chloroform at 37° for 12 h yields what is believed to be 1,2-bis(triphenylphosphite)-4,5,6-tricarbonyl-3-bromo-manganese(I)3, in which the P(OC6H5)3 groups are cis to the Br as well as to each other (the ‘cis-isomer’). However, reaction at 55° produces 1,6-bis(triphenylphosphite)-2,4,5-tricarbonyl-3-bromomanganese(I), in which the P(OC6H5)3 groups are cis to the Br but trans to each other (the ‘trans-isomer’). Both isomers can be precipitated from chloroform with n-hexane and analyse correctly for the above compounds. The dipole moments obtained in benzene by an approximate method4 were 4.8D for the cis-isomer and 3.5Dfor the trans, while Mn(CO)5Br itself had a moment of 3.4D. These measurements confirm the assignment of the trans structure, but do not distinguish between the possible isomers in which the phosphine ligands are (a) both cis to the bromide, or (b) one cis and one trans to the bromide. An attempt has been made to distinguish these by means of the nuclear magnetic resonance of phosphorus which should show splittings in case (b) owing to the different environments of the two ligands. Although resonance was observed, the resolution was poor and no conclusion could be reached on these grounds. (We thank Dr. J. Verkade and Dr. R. King, Iowa State University, for making these measurements.) However, in view of the known inertness5 of the CO trans to the bromide in Mn(CO)5Br, we feel that alternative (b) is unlikely.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Abel, E. W., and Wilkinson, G., J. Chem. Soc., 1501 (1959).
Angelici, R. J., and Basolo, F., J. Amer. Chem. Soc., 84, 2495 (1962).
Fernelius, W. C., Advances in Chemistry series, Amer. Chem. Soc., Easton, Pa., 8, 9 (1953).
Jensen, K. A., Acta Chem. Scand., 3, 479 (1949).
Wojcicki, A., and Basolo, F., J. Amer. Chem. Soc., 83, 525 (1961).
Verkade, J. G., and Reynolds, L. T., J. Org. Chem., 25, 663 (1960).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ANGELICI, R., BASOLO, F. & POË, A. Isomerism of Disubstituted Manganese Pentacarbonyl Bromide. Nature 195, 993–994 (1962). https://doi.org/10.1038/195993a0
Issue Date:
DOI: https://doi.org/10.1038/195993a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.