Abstract
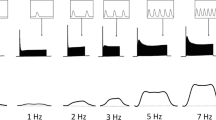
THE mechanism by which depolarisation of the muscle fibre membrane leads to release of stored Ca2+ ions from the sarcoplasmic reticulum, although crucial, is perhaps the least understood stage in excitation–contraction coupling1,2. We report here our investigation of the temperature dependence of this mechanism, using intracellular injection of arsenazo III (refs 3,4), a Ca2+ indicator dye with a fast response time5. We have found that the latency between depolarisation of the fibre and the onset of the rise in intracellular free Ca2+ is proportional to the reciprocal temperature, but that on an Arrhenius plot this relationship shows a change in slope at a temperature which depends upon the Ca2+ concentration in the bathing solution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ebashi, S. A. Rev. Physiol. 38, 293–313 (1976).
Endo, M. Physiol. Rev. 57, 71–108 (1977).
Parker, I. in Detection and Measurement of Free Calcium Ions in Cells (eds Ashley C. C. & Campbell A. K.) (Eisevier, Amsterdam, in the press).
Scarpa, A., Tiffert, T. & Brinley, F. J. in Biochemistry of Membrane Transport (eds Semenza G. & Carafoli E.) 552–566 (Springer, Berlin, 1977).
Brown, J. E. et al. Biophys. J. 15, 1155–1160 (1975).
Miledi, R., Parker, I. & Schalow, G. Proc. R. Soc. B198, 201–210 (1977).
Miledi, R., Parker, I. & Schalow, G. J. Physiol., Lond. 269, 11–13 (1977).
Suarez Kurtz, G. & Parker, I. Nature 270, 746–748 (1977).
Miledi, R., Parker, I. & Schalow, G. Nature 268, 750–752 (1977).
Gonzalez Serratos, H. J. Physiol., Lond. 212, 777–799 (1971).
Anderson, C. R., Cull-Candy, S. G. & Miledi, R. Nature 268, 663–665 (1977).
Dreyer, F., Muller, K. D., Peper, K. & Sterz, R. Pflügers Arch. Ges. Physiol. 367, 115–122 (1976).
Fischback, G. D. & Lass, Y. J. Physiol., Lond. 280, 527–536 (1978).
Feltz, A., Large, W. A. & Trautmann, A. J. Physiol., Lond. 269, 109–130 (1977).
Anderson, C. R., Cull-Candy, S. G. & Miledi, R. J. Physiol., Lond. 282, 219–242 (1978).
Chapman, D. Q. Rev. Biophys. 8, 185–235 (1975).
Wilson, G. & Fox, C. F. J. molec. Biol. 55, 49–60 (1971).
Overath, P. & Trauble, M. Biochemistry 12, 2625–2627 (1973).
Levy, H. M., Sharon, N. & Koshland, D. E. Proc. natn. Acad. Sci. U.S.A. 45, 785–791 (1959).
Dixon, M. & Webb, E. C. Enzymes 2nd edn, 758 (Longmans, London, 1964).
Caputo, C. J. Physiol., Lond. 255, 191–207 (1976).
Caputo, C. J. Physiol., Lond. 223, 483–505 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MILEDI, R., PARKER, I. & SCHALOW, G. Transition temperature of excitation–contraction coupling in frog twitch muscle fibres. Nature 280, 326–328 (1979). https://doi.org/10.1038/280326a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/280326a0
This article is cited by
-
Calcium transients in skeletal muscle fibres under isometric conditions and during and after a quick stretch
Journal of Muscle Research and Cell Motility (1991)
-
Characterization of the mobile charges in the membrane ofValonia utricularis
The Journal of Membrane Biology (1985)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.