Abstract
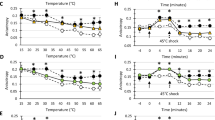
THE use of lacZ gene fusions, producing a hybrid protein containing an amino terminus specified by a target gene fused to the functional carboxy terminus of β-galactosidase, has facilitated the study of protein targeting in various organisms. One of the best characterized fusions in Eschcrichia coli is Φ(lamB–lacZ)42-l(Hyb), which produces a hybrid protein with the signal sequence and 181 N-terminal amino acids of the exported protein LamB, attached to LacZ1. In common with other LacZ hybrids (reviewed in ref. 2), the LamB–LacZ(42-l) protein is poorly exported from E. coli, conferring a Lac+ phenotype. β-Galactosidase activity decreases markedly when cells producing the LamB–LacZ protein are grown at 42 ° C or when a heat-shock response is induced at lower temperatures by overproducing heat-shock factor RpoH3, indicating the LacZ hybrids are being efficiently targeted to the cell envelope. We now report that the heat-shock proteins DnaK and GroEL can, in sufficient amounts, decrease β-galactosidase activity and facilitate the export of lacZ-hybrid proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Silhavy, T. J., Shuman, H. A., Beckwith, J. & Schwartz, M. Proc. natn. Acad. Sci. U.S.A. 74, 5411–5414 (1977).
Bieker, K. L., Phillips, G. J. & Silhavy, T. J. J. Bioenergetics Biomembranes (in the press).
Grossman, A. D., Straus, D. B., Walter, W. A. & Gross, C. A. Genes Dev. 1, 179–184 (1987).
Benson, S. A., Bremer, E. & Silhavy, T. J. Proc. natn. Acad. Sci. U.S.A. 81, 3820–3834 (1984).
Laemmli, U. K. Nature 227, 680–685 (1970).
Chandrasekhar, G. N., Tilly, K., Woolford, C., Hendrix, R. & Georgopoulos, C. J. biol. Chem. 261, 12414–12419 (1986).
Georgopoulos, C. P. & Hohn, B. Proc. natn. Acad. Sci. U.S.A. 75, 131–135 (1978).
Tilly, K., Murialdo, H. & Georgopoulos, C. Proc. natn. Acad. Sci. U.S.A. 78, 1629–1633 (1981).
Hemmingsen, S. M. et al. Nature 333, 330–334 (1988).
Bochkareva, E. S., Lissin, N. M. & Girshovich, A. S. Nature 336, 254–257 (1988).
Lecker, S. et al. EMBO J. 8, 2703–2709 (1989).
Kusukawa, N., Yura, T., Ueguchi, C., Akiyama, Y. & Ito, K. EMBO J. 8, 3517–3521 (1989).
Deshaies, R. J., Koch, B. D., Werner-Washburne, M., Craig, E. A. & Schekman, R. Nature 332, 800–805 (1988).
Chirico, W. J., Waters, M. G. & Blobel, G. Nature 332, 805–810 (1988).
Murakami, H., Pain, D. & Blobel, G. J. Cell Biol. 107, 2051–2057 (1988).
Bardwell, J. C. A. & Craig, E. A. Proc. natn. Acad. Sci. U.S.A. 81, 848–852 (1984).
Lee, C., Li, P., Inouye, H., Brickman, E. R. & Beckwith, J. J. Bact. 171, 4609–4616 (1989).
Rothman, J. E. Cell 59, 591–601 (1989).
Miller, J. Experiments in Molecular Genetics (Cold Spring Harbor Laboratories, Cold Spring Harbor, New York, 1984).
Silhavy, T. J., Berman, M. L. & Enquist, L. W. Experiments with Gene Fusions (Cold Spring Harbor Laboratories, Cold Spring Harbor, New York, 1984).
Fayet, O., Louarn, J.-M. & Georgopoulos, C. Molec. gen. Genet. 202, 435–445 (1986).
Zylicz, M. & Georgopoulos, C. J. biol. Chem. 259, 8820–8825 (1984).
Stader, J., Benson, S. A. & Silhavy, T. J. J. biol. Chem. 261, 15075–15080 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Phillips, G., Silhavy, T. Heat-shock proteins DnaK and GroEL facilitate export of LacZ hybrid proteins in E. coli. Nature 344, 882–884 (1990). https://doi.org/10.1038/344882a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/344882a0
This article is cited by
-
Multitasking of Hsp70 chaperone in the biogenesis of bacterial functional amyloids
Communications Biology (2018)
-
Molecular chaperones, folding catalysts, and the recovery of active recombinant proteins fromE. coli
Applied Biochemistry and Biotechnology (1997)
-
Construction of a vector for probing the effect of co-expression of dnaY and secB upon secreted gene products in Escherichia coli
Biotechnology Techniques (1995)
-
Expression of one of the members of the Arabidopsis chaperonin 60? gene family is developmentally regulated and wound-repressible
Plant Molecular Biology (1994)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.