Abstract
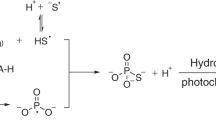
The Precambrian banded iron formations (BIFs) are the major iron ore sources on the Earth. They consist of extensive iron-rich and iron-poor layers within siliceous sedimentary rocks1,2. The banding has been attributed to variations in the conditions for precipitation of Fe2+ in ancient seas. The most favoured precipitating agent is oxygen3–8; this would lead in the first place to insoluble FeOOH. The variations might then arise from fluctuations in low levels of oxygen in the atmosphere8. Similar fluctuations could arise through the in situ photosynthetic activity of phytoplankton5. Alternatively, oxygen might have been a constant factor, the periodicity arising from a varying supply of iron to the zone of precipitation8–10. Another suggestion, that UV photons might have been the precipitating agent, without oxygen11, was based on the conversion of Fe2+ to Fe3+ by UV light (254 nm) in rather strongly acid conditions12,13. We have now tested this idea by photolysing Fe2+ in morerealistic near-neutral conditions. We report here that the presence of Fe(OH)+ becomes important, greatly enhancing previous estimates of the iron-precipitating power of early Precambrian sunlight, and suggesting that this sunlight would have been a sufficient precipitating agent for the iron found in BIFs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
James, H. L. & Sims, P. K. Econ. Geol. 68, 913–914 (1973); 915–1179 (1973).
Trendall, A. F. & Blockley, J. G. Bull. geol. Surv. West. Aust. 119 (1970).
Macgregor, A. M. S. Afr. J. Sci. 24, 155–172 (1927).
Cloud, P. E. Science 160, 729 (1968).
Cloud, P. E. Econ. Geol. 68, 1135–1143 (1973).
Schidlowski, M. in The Early History of the Earth (ed. Windley, B. F.) 525–535 (Wiley, London, 1976).
Schopf, J. W. A. Rev. Earth planet. Sci. 3, 213–249 (1975).
Towe, K. M. Nature 274, 657–661 (1978).
Holland, H. D. Econ. Geol. 68, 1169–1172 (1973).
Brock, T. D. Nature 288, 214 (1980).
Cairns-Smith, A. G. Nature 276, 807–808 (1970).
Weiss, J. Nature 136, 794 (1935).
Jortner, J. & Stein, G. J. phys. Chem. 66, 1258–1271 (1962).
Hayon, E. & Weiss, J. J. chem. Soc. 3866 (1960).
Garrels, R. M. & Perry, E. A. in The Sea Vol. 5 (ed. Goldberg, E. G.) 303–336 (Wiley, London, 1974).
James, H. L. Econ. Geol. 49, 235–203? (1954).
Baes, C. F. & Mesmer, R. E. The Hydrolysis of Cations (Wiley, New York, 1976).
Ehrenfreud, M. & Leibenguth, J.-L. Bull. Soc. chim. Fr. 2494–2505 (1970).
Weast, R. C. (ed.) CRC Handbk Chem. Phys. 61st edn (1980–81).
Canuto, V. M., Levine, J. S., Augustsson, T. R. & Imhoff, C. L. Nature 296, 816–820 (1982).
Kolthoff, I. M. & Sandell, E. B. (eds) Textbook of Quantitative Inorganic Analysis 3rd edn (Macmillan, New York, 1965).
Hatchard, C. G. & Parker, C. A. Proc. R. Soc. A235, 518–536 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Braterman, P., Cairns-Smith, A. & Sloper, R. Photo-oxidation of hydrated Fe2+—significance for banded iron formations. Nature 303, 163–164 (1983). https://doi.org/10.1038/303163a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/303163a0
This article is cited by
-
Genesis: early life survived in the Polar Circles by precipitating banded iron formation (~ 3.7–1.85 Ga) followed by stratified ferruginous siliciclasts until ~ 580 Ma, when tectonically shifted to lower latitudes initiating the ‘Cambrian Explosion’
International Journal of Earth Sciences (2022)
-
Microbial processes during deposition and diagenesis of Banded Iron Formations
PalZ (2021)
-
Role of the Interchangeable Cations on the Sorption of Fumaric and Succinic Acids on Montmorillonite and its Relevance in Prebiotic Chemistry
Origins of Life and Evolution of Biospheres (2021)
-
The Effect of Goethites on the Polymerization of Glycine and Alanine Under Prebiotic Chemistry Conditions
Origins of Life and Evolution of Biospheres (2021)
-
Unexpected Thiocyanate Adsorption onto Ferrihydrite Under Prebiotic Chemistry Conditions
Origins of Life and Evolution of Biospheres (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.