Abstract
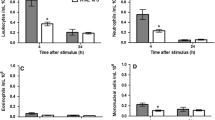
Based on observations obtained by the use of intravital microscopy, we report that prostaglandins, (PGs) can exert inhibitory effects on mast cell-dependent inflammation. Thus, the PG-synthesis inhibitors diclofenac and indomethacin potentiated extravasation of plasma evoked by challenge with the mast cell secretagogue compound 48/80. Although the plasma leakage induced by compound 48/80 was in large mediated by histamine, neither diclofenac nor indomethacin potentiated the plasma leakage caused by exogenous histamine. These findings indicated that endogenous PGs inhibited the mast cell-dependent reaction at the level of mediator release. This mode of action was confirmed, as diclofenac was found to enhance thein vivo release of histamine that ensued challenge with compound 48/80. Moreover, the enhancement of the response to compound 48/80 observed after diclofenac treatment was prevented by local administration of PGE2 (30 nM). This inhibition included both the histamine release and the plasma leakage. In addition, diclofenac enhanced the leukocyte emigration after compound 48/80 challenge, and PGE2 reversed also this effect, suggesting that endogenous PGs (e.g. PGE2) also inhibited the release of chemotactic, mediators.
Similar content being viewed by others
References
J. Raud, S.-E. Dahlén, A. Sydbom, L. Lindbom and P. Hedqvist,Enhancement of acute allergic inflammation by indomethacin is reversed by prostaglandin E 2:Apparent correlation with in vivo modulation of mediator release. Proc. Natl. Acad. Sci. USA85, 2315–2319 (1988).
J. Raud, S.-E. Dahlén, G. Smedegård and P. Hedqvist,An intravital microscopic model for mast cell-dependent inflammation in the hamster cheek pouch. Acta Physiol. Scand.135, 95–105 (1989).
J. Raud, L. Lindbom, S.-E. Dahlén and P. Hedqvist.Periarteriolar localization of mast cells promotes oriented interstitial migration of leukocytes in the hamster cheek pouch. Am. J. Pathol.134, 161–169 (1989).
W. D. M. Paton,Compound 48/80: A potent histamine liberator. Br. J. Pharmacol.6, 499–508 (1951).
J. Björk, G. Smedegård, E. Svensjö and K.-E. Arfors,The use of the hamster cheek pouch for intravital microscopy studies of microvascular events. Prog. Appl. Microcirc.6, 41–53 (1984).
E. Svensjö, K.-E. Arfors, G. Arturson and G. Rutili,The hamster cheek pouch preparation as a model for studies of macromolecular permeability of the microcirculation. Upsala J. Med. Sci.83, 71–79 (1978).
B.R. Duling,The preparation and use of the hamster cheek pouch for studies of the microcirculation. Microvasc. Res.,5, 423–429, (1973).
P.A. Shore, A. Burkhalter and V.H. Cohn,A method for the fluorometric assay of histamine in tissues. J. Pharmacol. Exp. Ther.127, 182–186 (1959).
L.M. Lichtenstein and H.R. Bourne,Inhibition of allergic histamine release by histamine and other agents which stimulate adenyl cyclase. InBiochemistry of Acute Allergic Reactions (Ed. K.F. Austen and E.L. Becker) pp. 161–174, Blackwell, Oxford, 1971.
J.L. Walker,The regulatory function of prostaglandins in the release of histamine and SRS-A from passively sensitized human lung tissue. InAdvances in the Biosciences, vol. 9. (Eds. S. Bergström and S. Bernhard) pp. 235–240, Pergamon Press, Vieweg 1973.
M. Kaliner and K.F. Austen,Cyclic AMP, ATP, and reversed anaphylactic histamine release from rat mast cells. J. Immunol.112, 664–674 (1974).
M. Hitchocock,Effect of inhibitors of prostaglandin synthesis and prostaglandin E 2 and F2α on the immunologic release of mediators of inflammation from actively sensitized guinea-pig lung. J. Pharmacol. Exp. Ther.207, 630–640 (1978).
E.A. Ham, D.D. Soderman, M.E. Zanetti, H.W. Dougherty, E. McCauley and F.A. Kuehl J.R.,Inhibition by prostaglandins of leukotriene B 4 release from activated neutrophils. Proc. Natl. Acad. Sci. USA80, 4349–4353 (1983).
T.H. Lee, E. Israel, J.M. Drazen, A.G. Leitch, J. Ravalese III, E.J. Corey, D.R. Robinson, R.A. Lewis and K.F. Austen,Enhancement of plasma levels of biologically active leukotriene B compounds during anaphylaxis in guinea pigs pretreated by indomethacin or by a fish oil-enriched diet. J. Immunol.136, 2575–2582 (1986).
R. Levi, G. Allan and H. Zavecz,Prostaglandins and cardiac acaphylaxis. Life Sci.18, 1255–1264 (1976).
T. Okazaki, V.S. Ilea, N.A. Rosario, R.E. Reisman, C.E. Arbesman, J.B. Lee and E. Middleton Jr,Regulatory role of prostaglandin, E in allergic histamine release with observations on the responsiveness of basophil leukocytes and the effect of acetylsalicylic acid. J. Allergy Clin. Immunol.60, 360–366 (1977).
D.M. Engineer, U. Niederhauser, P.J. Piper and P. Sirois,Release of mediators of anaphylaxis: Inhibition of prostaglandin synthesis and the modification of release of slow reacting substance of anaphylaxis and histamine. Br. J. Pharmacol.62, 61–66 (1978).
T.J. Williams,Interactions between prostaglandins, leukotrienes and other mediators of inflammation. Br. Med. Bull.39, 239–242 (1983).
S.H. Ferreira and J.R. Vane,Mode of action of antiinflammatory agents which are prostaglandin synthetase inhibitors. InAnti-Inflammatory Drugs (Eds J.R. Vane and S.H. Ferreira) pp. 348–398, Springer, Berlin 1979.
G.A. Higgs, K.E. Eakins, K.G. Mugridge, S. Moncada and J.R. Vane,The effects of non-steroid anti-inflammatory drugs on leukocyte migration in carrageenin-induced inflammation. Eur. J. Pharmacol.66, 81–86 (1980).
P. Hedqvist, S.-E. Dahlén and U. Palmertz,Leukotriene dependent airway anaphylaxis in guinea pigs. Prostaglandins28, 605–608 (1984).
C.N. Ellis, J.D. Fallon, S. Kang, E.E. Vanderveen and J.J. Voorhees,Topical application of nonsteroidal anti-inflammatory drugs prevents vehicle-induced improvement of psoriasis. J. Am. Acad. Dermatol.14, 39–43 (1986).
J. Björk and G. Smedegard,Immune-complex-induced, inflammatory reaction studied by intravital microscopy: Role of histamine and arachidonic acid metabolites. Inflammation11, 47–58 (1987).
C. Lundberg and B. Gerdin,The inflammatory reaction in an experimental model of open wounds in the rat. The effect of arachidonic acid metabolites. Eur. J. Pharmacol.97, 229–238 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raud, J., Sydbom, A., Dahlén, S.E. et al. Prostaglandin E2 prevents diclofenac-induced enhancement of histamine release and inflammation evoked byin vivo challenge with compound 48/80 in the hamster cheek pouch. Agents and Actions 28, 108–114 (1989). https://doi.org/10.1007/BF02022990
Issue Date:
DOI: https://doi.org/10.1007/BF02022990