Abstract
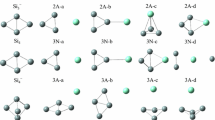
Various structural possibilities for Si3C3 clusters are investigated by ab initio calculations employing basis sets of double- and triple-zeta quality augmented by d polarization functions. Correlation effects are included by a second-order Moeller Plesset perturbation treatment. For the two lowest-lying structures higher-order correlation corrections and multi-reference effects are also included. Bonding features are investigated by two different types of population analyses to obtain insight into the nature of chemical bonding. A total of 17 stationary points were investigated, 14 of which correspond to local minima and three being transition states. The energetically lowest-lying structures are: A “pyramidlike” structure with various multicenter bonds, followed by a Cs symmetric isomer closely related to the ground state Si6 structure. Planar structures, favoured in small carbon clusters, lie higher in energy and are transition states. The lowest-lying triplet system is found to be the linear nonsymmetric Si-C-C-C-Si-Si structure, which is calculated to lie about 38 kcal/mole above the singlet ground state. A building-up principle based on bonding criteria is suggested for the occurence of the various structural possibilities.
Similar content being viewed by others
References
Parasuk, V., Almlöf, J.: J. Chem. Phys.94, 8172 (1991)
Bernholdt, D.E., Magers, D.H., Bartlett, R.J.: J. Chem. Phys.89, 3612 (1988)
Martin, J.M.L., Francoise, J.P., Gijbels, R.: J. Chem. Phys.94, 3753 (1991)
Raghavachari, K., Binkley, J.S.: J. Chem. Phys.84, 2191 (1987)
Martin, J.M.L., Francoise, J.P., Gijbels, R.: J. Comp. Chem.12, 52 (1991)
Parasuk, V., Almlöf, J.: J. Chem. Phys.91, 1137 (1989)
Ragavashari, K., Whiteside, R.A., Pople, J.A.: J. Chem. Phys.85, 6623 (1986)
Weltner Jr., W., Van Zee, R.J.: Chem. Rev.89, 1714 (1989)
Anderson, L.R., Maruyama, S., Smalley, R.E.: Chem. Phys. Lett.176, 348 (1991)
Raghavachari, K.: In: Phase transitions, Vols. 24–26, pp. 61–69 (1990)
Raghavachari, K., Rohlfing, C.: J. Chem. Phys.94, 3670 (1991)
Raghavachari, K., Rohlfing, C.: Chem. Phys. Lett.167, 559 (1990)
Raghavachari, K.: J. Chem. Phys.84, 5672 (1986)
Presilla-Marquez, J.D., Graham, W.R.M.: J. Chem. Phys.96, 6509 (1992)
Rittby, C.M.L.: J. Chem. Phys.96, 6768 (1992)
Trucks, G.W., Bartlett, J.R.: J. Mol. Struct. (Theochem)135, 423 (1986)
Sudhakar, V., Günner, O.F., Lammertsma, K.: J. Phys. Chem.93, 7289 (1989)
Lammertsma, K., Günner, O.F.: J. Am. Chem. Soc.110, 5239 (1988)
Huzinaga, S., Sakai, Y.: J. Chem. Phys.50, 1371 (1969); Dunning Jr., T.H., Hay, P.J.: Modern theoretical chemistry, Vol. 3, Chap. 1. Schaefer III, H.F. (ed.). New York: Plenum Press 1977
Hehre, W.J., Radom, L., Schleyer, P.v.R., Pople, J.A.: Ab inito molecular orbital theory. New York: Wiley 1985
Ahlrichs, R., Bär, M., Häser, M., Horn, H.: Chem. Phys. Lett.162, 165 (1989)
Häser, M., Ahlrichs, R.: J. Comput. Chem.10, 104 (1989)
Woon, D.E., Dunning Jr., T.H., Harrison, R.J.: J. Chem. Phys.96, 6796 (1992)
Dunning Jr., T.H.: J. Chem. Phys.90, 1007 (1989)
Anderson, K., Malmqvist, R.Å., Roos, B.O., Sadlej, A.J., Wolinski, K.: J. Phys. Chem.94, 5483 (1990)
Anderson, K., Malmqvist, R.Å., Roos, B.O.: J. Chem. Phys.96, 1218 (1992)
Mulliken, R.S.: J. Chem. Phys.23, 1833 (1955)
Davidson, E.R.: J. Chem. Phys.46, 3320 (1967)
Heinzmann, R., Ahlrichs, R.: Theor. Chim. Acta42, 33 (1976)
Roby, K.R.: Mol. Phys.27, 81 (1974)
Ehrhardt, C., Ahlrichs, R.: Theor. Chim. Acta168, 231 (1985)
Mühlhäuser, M., Froudakis, G., Zdetsis, A., Peyerimhoff, S.D.: Chem. Phys. Lett.204, 617 (1993)
Grev, R.S., Schaefer III, H.F.: J. Chem. Phys.82, 4126 (1985)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mühlhäuser, M., Froudakis, G., Zdetsis, A. et al. Ab initio investigation of the stability of Si3C3 clusters and their structural and bonding features. Z Phys D - Atoms, Molecules and Clusters 32, 113–123 (1994). https://doi.org/10.1007/BF01425931
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01425931