Abstract
Titania nanoparticles have been produced by the controlled hydrolysis of tetraisopropyltitanate (TPT) in sodium bis(2-ethylhexyl)sulfosuccinate (AOT) reverse micelles. Particle formation and aggregation were investigated by static and dynamic light scattering and the chemical species by vibrational spectroscopy.
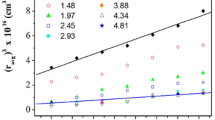
The kinetics of particle formation and aggregation were controlled by varying [H2O]/[AOT] (w 0), [H2O]/[Ti(IV)] and [AOT]/[Ti(IV)]. Nanoparticles, with diameters<10 nm, could be produced at relatively high Ti(IV) concentrations (up to 0.05 M). These nanoparticles aggregated into sols, with colloid sizes of 20 to 200 nm, eventually forming gelatinous precipitates.
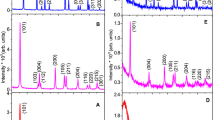
Different titania phases were produced, depending on the size of the micellar water pool; small pools (w 0<6) yielded amorphous particles, while larger pools (w 0>10) produced anatase.
Similar content being viewed by others
References
R.W. Siegel and J.A. Eastman, Mater. Res. Symp. Proc.132, 3 (1988).
P.D.I. Fletcher, A.M. Howe, and B.H. Robinson, J. Chem. Soc., Faraday Trans. I83, 985 (1987).
K. Osseo-Asare and F.J. Arriagada, in Ceramic Transactions12 (1990) (Proc. 3rd Int. Conf. on Powder Processing Science, edited by G.L. Messing et al., San Diego, Feb. 4–6, 1990) p.3.
H. Yamauchi, T. Ishikawa, and S. Kondo, Colloids Surf.37, 71 (1989).
T. Hirai, H. Sato, and I. Komasawa, Ind. Eng. Chem. Res.32, 3014 (1993).
C.J. Brinker and G.W. Scherer,Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing (Academic Press, San Diego, 1990).
J.R. Bartlett and J.L. Woolfrey, Mater. Res. Symp. Proc.271, 309 (1992).
P.D. Moran, G.A. Bowmaker, R.P. Cooney, J.R. Barlett, and J.L. Woolfrey, Langmuir11, 738 (1995).
J.C. Parker and R.W. Siegel, J. Mater. Res.5, 1246 (1990).
J.R. Bartlett, M.J. Percy, J.L. Woolfrey, L. Spiccia, and B.O. West, J. Sol-Gel Sci. Technol.2, 215 (1994).
A. Maitra, J. Phys. Chem.88, 5122 (1984).
S. Tohno and M. Itoh, J. Aerosol Sci.24, 339 (1993).
J.P. Wilcoxon, R.L. Williamson, and Baughman, J. Chem. Phys.98, 9933 (1993).
P. Plucinski and W. Nitsch, Langmuir10, 371 (1994).
R. Jóhannsson, M. Almgren, and J. Alsins, J. Phys. Chem.95, 3819 (1991).
B. Boddenberg and W. Horstmann, Ber. Bunsenges. Phys. Chem.92, 519 (1988).
D.N.L. McGown, G.D. Parfitt, and E. Willis, J. Colloid Sci.20, 650 (1965).
H. Yotsumoto and R.-H. Yoon, J. Colloid Interface Sci.157, 426 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moran, P.D., Bartlett, J.R., Woolfrey, J.L. et al. Formation and gelation of titania nanoparticles from AOT reverse micelles. J Sol-Gel Sci Technol 8, 65–69 (1997). https://doi.org/10.1007/BF02436819
Issue Date:
DOI: https://doi.org/10.1007/BF02436819