Summary
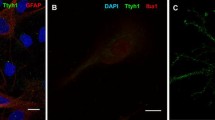
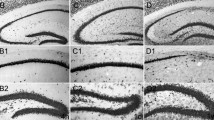
Kainic acid treatment, a model of temporal lobe epilepsy, induces Ammon's horn sclerosis characterized by degeneration of CA3 pyramidal neurons and reactive gliosis. We now report that in kainic acid treated rats, reactive astrocytes in the hippocampus are A2B5 immunopositive and express GAP-43 immunoreactivity. A2B5 is a cell surface ganglioside selectively expressed in the glial O-2A lineage (oligodendrocytes and type-2 astrocytesin vitro). Since A2B5-positive cells were also GFAP immunoreactive, our observations suggest that hippocampal-reactive astrocytes in the epileptic process are type-2 astrocytes.
GAP-43 is a membrane-associated phosphoprotein involved in neurite outgrowth.In vitro analysis showed that the glial O-2A lineage may express this phosphoprotein. In this study, we found that GAP-43 was coexpressed in astrocytes with A2B5 suggesting thatin vivo asin vitro type-2 astrocytes express GAP-43.
Similar content being viewed by others
References
Albe-Fessard, D., Stutinski, F. &Libouban-Le-Touze, S. (1971)Atlas stéréotaxique du diencephale du rat blanc. Paris: CNRS.
Anderson, P.-B., Perry, V. H. &Gordon, S. (1991) The kinetics and morphological characteristics of the macrophage-microglial response to kainic acid-induced neuronal degeneration.Neuroscience 42, 201–14.
Baudier, J., Bronner, C., Kligman, D. &Cole, R. D. (1989) Protein kinase C substrates from bovine brain: purification and characterization of neuromodulin, a neuron-specific calmodulin-binding protein.Journal of Biological Chemistry 264, 1824–8.
Ben-Ari, Y. (1985) Limbic seizures and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy.Neuroscience 14, 375–403.
Ben-Ari, Y., Tremblay, E., Ottersen, O. P. &Naquet, R. (1979) Evidence suggesting secondary epileptogenic lesion after kainic acid: pretreatment with diazepam reduces distant but not local brain damage.Brain Research 165, 362–5.
Ben-Ari, Y., Tremblay, E. &Ottersen, O. P. (1980) Injection of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of the epilepsy.Neuroscience 5, 515–28.
Benowitz, L. I., Perrone-Bizzozero, N. I. &Finkle-Stein, S. P. (1987) Molecular properties of the growth-associated protein GAP-43 (B-50).Journal of Neurochemistry 48, 1640–7.
Biffo, S., Verhaagen, J., Schrama, L. H., Schotman, P., Danho, W. &Margolis, F. L. (1990) B-50/GAP-43 expression correlates with process outgrowth in the embryonic mouse nervous system.European Journal of Neuroscience 2, 487–99.
Crépel, V., Represa, A., Beaudoin, M. &Ben-Ari, Y. (1989) Hippocampal damage induced by ischemia and intra-amygdaloid kainate injection: effects on NMDA, TCP and glycine binding sites.Neuroscience 31, 605–12.
Curtis, R., Hardy, R., Reynolds, R., Spruce, B. A. &Wilkin, G. P. (1991) Down-regulation of GAP-43 during oligodendrocyte development and lack of expression by astrocytesin vivo: implications for macroglial differentiation.European Journal of Neuroscience 3, 876–86.
David, S., Miller, R. H., Patel, R. &Raff, M. C. (1984) Effects of neonatal transection on glial cell development in the rat optic nerve: evidence that the oligodendrocyte-type 2 astrocyte cell lineage depends on axons for its survival.Journal of Neurocytology 13, 961–74.
Deloulme, J. C., Janet, T., Au, D., Storm, D. R., Sensen-Brenner, M. &Baudier, J. (1990) Neuromodulin (GAP43): a neuronal protein kinase C substrate is also present in 0–2A glial cell lineage. Characterization of neuromodulin in secondary cultures of oligodendrocytes and comparison with the neuronal antigen.Journal of Cell Biology 111, 1559–69.
Dubois-Dalcq, M. (1987) Characterization of a slowly proliferative cell along the oligodendrocyte pathway.EMBO Journal 6, 2587–95.
Dusart, I., Marty, S. &Peschanski, M. (1991) Glial changes following an excitotoxic lesion in the CNS-II. Astrocytes.Neuroscience 45, 541–9.
Godfraind, C., Friedrich, V. L., Holmes, K. V. &Dubois-Dalcq, M. (1989)In vivo analysis of glial cell phenotypes during a viral demyelinating disease in mice.Journal of Cell Biology 109, 2405–16.
Goslin, K., Schreyer, D. J., Skene, J. H. P. &Banker, G. (1988) Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones.Nature 336, 672–4.
Jacobson, R. D., Virag, I. &Skene, J. H. P. (1986) A protein associated with axon growth, GAP-43 is widely distributed and developmentally regulated in rat CNS.Journal of Neuroscience 6, 1843–55.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature 227, 680–5.
Levine, S. M., Seyfried, T. N., Yu, R. K. &Goldman, J. E. (1986) Immunocytochemical localization of GD3 ganglio-side to astrocytes in murine cerebellar mutants.Brain Research 374, 260–9.
Lowry, O. H., Rosebrough, N. J., Farr, A. L. &Randall, R. J. (1951) Protein measurement with the folin phenol reagent.Journal of Biological Chemistry 193, 265–72.
Marty, S., Dusart, I. &Peschanski, M. (1991) Glial changes following an excitotoxic lesion in the CNS-I. Microglia/macrophages.Neuroscience 45, 529–39.
McGuire, C. B., Snipes, G. J. &Norden, J. J. (1988) Light-microscopic immunolocalization of the growth- and plasticity-associated protein GAP-43 in the developing rat brain.Developmental Brain Research 41, 277–91.
Meiri, K. F. &Gordon-Weeks, P. R. (1990) Gap-43 in growth cones is associated with areas of membrane that are tightly bound to substrate and is a component of a membrane skeleton subcellular fraction.Journal of Neuroscience 10, 256–66.
Miller, R. H., David, S., Patel, R., Abney, E. R. &Raff, M. C. (1985) A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve:in vivo evidence for two distinct astrocyte lineages.Developmental Biology 111, 35–41.
Moss, D. J., Fernyhough, P., Chapman, K., Baizer, L., Bray, D. &Allsopp, T. (1990) Chicken growth-associated protein GAP-43 is tightly bound to the actinrich neuronal membrane skeleton.Journal of Neurochemistry 54, 729–36.
Nadler, J. V., Perry, B. W., Gentry, C. &Cotman, C. W. (1980) Loss and reacquisition of hippocampal synapses after selective destruction of CA3-CA4 afferents with kainic acid.Brain Research 191, 387–403.
Neve, R. L., Perrone-Bizzozero, N. I., Finklestein, S., Zwiers, H., Bird, E., Kurnit, D. M. &Benowitz, L. I. (1987) The neuronal growth-associated protein GAP-43 (B50, F1): neuronal specificity, developmental regulation and regional distribution of the human and rat mRNAs.Molecular Brain Research 2, 177–83.
Oestreicher, A. B. &Gispen, W. H. (1986) Comparison of the immunocytochemical distribution of the phospho-protein B-50 in the cerebellum and hippocampus of immature and adult rat brain.Brain Research 375, 267–79.
Okazaki, M. M. &Nadler, J. V. (1988) Protective effects of mossy fiber lesions against kainic acid-induced seizures and neuronal degeneration.Neuroscience 26, 763–81.
Raff, M. C. (1989) Glial cell diversification in the rat optic nerve.Science 243, 1450–5.
Raff, M. C., Miller, R. H. &Noble, M. (1983) A glial progenitor cell that developsin vitro into astrocyte or an oligodendrocyte depending on culture medium.Nature 303, 390–6.
Represa, A., Tremblay, E. &Ben-Ari, Y. (1987) Kainate binding sites in the hippocampal mossy fibers: localization and plasticity.Neuroscience 20, 739–48.
Shea, T. B., Perrone-Bizzozero, N. I., Beermann, M. L. &Benowitz, L. I. (1991) Phospholipid-mediated delivery of anti-GAP-43 antibodies into neuroblastoma cells prevents neuritogenesis.Journal of Neuroscience 11, 1685–90.
Skene, J. H. P. (1989) Axonal growth-associated proteins.Annual Review of Neuroscience 12, 127–56.
Tauck, D. L. &Nadler, J. V. (1985) Evidence for functional mossy fiber sprouting in hippocampal formation of kainic acid treated rats.Journal of Neuroscience 5, 1016–22.
Van Lookeren Campagne, M., Oestreicher, A. B., Van Bergen En Henegouwen, P. M. P. &Gispen, W. H. (1990) Ultrastructural double localization of B50/GAP-43 and synaptophysin (p38) in the neonatal and adult rat hippocampus.Journal of Neurocytology 19, 948–61.
Vayssen, P. J.-J. &Goldman, J. E. (1992) A distinct type of GD3+, flat astrocyte in rat CNS cultures.Journal of Neuroscience 12, 330–70.
Vitkovic, L., Steisslinger, H. W., Aloyo, V. J. &Mersel, M. (1988) The 43-kDa neuronal growth-associated protein (GAP-43) is present in plasma membranes of rat astrocytes.Proceedings of the National Academy of Sciences (USA) 85, 8296–300.
Yamamoto, M., Marshall, P., Hemmendinger, L. M., Boyer, A. B. &Caviness, V. S. Jr. (1988) Distribution of glucuronic acid- and -sulfate-containing glycoproteins in the central nervous system of the adult mouse.Neuroscience Research 5, 273–98.
Zuber, M. X., Goodman, D. W., Karns, L. R. &Fishman, M. C. (1989) The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells.Science 244, 1193–5.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Represa, A., Niquet, J., Charriaut-Marlangue, C. et al. Reactive astrocytes in the kainic acid-damaged hippocampus have the phenotypic features of type-2 astrocytes. J Neurocytol 22, 299–310 (1993). https://doi.org/10.1007/BF01187128
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01187128