Summary
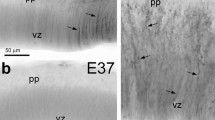
The differentiation of glia in the central nervous system is not well understood. A major problem is the absence of an objective identification system for involved cells, particularly the early-appearing radial glia. The intermediate filament structural proteins vimentin and glial fibrillary acidic protein have been used to define the early and late stages, respectively, of astrocyte development. However, because of the non-specificity of vimentin and the temporal overlap in expression patterns of both proteins, it is difficult to refine our view of the process. This is especially true of the early differentiation events involving radial glia. Using the developmentally-expressed intermediate filament-associated protein IFAP-70/280 kD in conjunction with vimentin and glial fibrillary acidic protein markers, a comprehensive investigation of this problem was undertaken using immunofluorescence microscopy of developing rat spinal cord (E13-P28 plus adult). The phenotypes of the cells were defined on the basis of their immunologic composition with respect to IFAP-70/280 kD (I), vimentin (V) and GFAP (G). A definitive immunotype for radial glia was established, viz, I+/V+/G−; thus reliance upon strictly morphological criteria for this early developmental cell was no longer necessary. Based upon the immunotypes of the cells involved, four major stages of macroglial development were delineated: (1) radial glia (I+/V+/G−); (2) macroglial progenitors (I+/V+/G+); (3) immature macroglia (I−/V+/G+); and (4) mature astrocytes (I−/V+/G+ primarily in white matter and I−/V−/G+, the predominant type in gray matter). It is of interest to note that the cells of the floor plate were distinguished from radial glia by their lack of IFAP-70/280 kD immunoreactivity. Introduction of the IFAP-70/280 kD marker has therefore provided a more refined interpretation of the various differentiation stages from radial glia to mature astrocytes.
Similar content being viewed by others
References
Abd-El-Basset, E. M., Kalnins, V. I., Subrahmanyan, L., Ahmed, I. &Fedoroff, S. (1988a) 48-kilodalton intermediate-filament-associated protein in astrocytes.Journal of Neuroscience Research 19, 1–13.
Abd-El-Basset, E. M., Kalnins, V. I. &Fedoroff, S. (1988b) Expression of 48-kilodalton intermediate filament-associated protein in differentiating and in mature astrocytes in various regions of the central nervous system.Journal of Neuroscience Research 21, 226–37.
Albers, K. &Fuchs, E. (1992) The molecular biology of intermediate filament proteins.International Review of Cytology 134, 243–79.
Altman, J. &Bayer, S. A. (1984)The development of the rat spinal cord Berlin: Springer-Verlag.
Bignami, A. &Dahl, D. (1974) Astrocyte-specific protein and neuroglial differentiation: an immunofluorescence study with antibodies to the glial fibrillary acidic protein.Journal of Comparative Neurology 153, 27–38.
Bignami, A., Eng., L. F., Dahl, D. &Uyeda, C. T. (1972) Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence.Brain Research 43, 429–35.
Bignami, A., Raju, T. &Dahl, D. (1982) Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons.Developmental Biology 91, 286–95.
Bongcam-Rudloff, E., Nister, M., Betsholtz, C., Wang, J.-L., Stenman, G., Huebner, K., Croce, C. M. &Westermark, B. (1991) Human glial fibrillary acidic protein: Complementary DNA cloning, chromosome localization, and messenger RNA expression in human glioma cell lines of various phenotypes.Cancer Research 51, 1553–60.
Bovolenta, P., Liem, R. K. &Mason, C. A. (1984) Development of cerebellar astroglia: transitions in form and cytoskeletal content.Developmental Biology 102, 248–59.
Carnow, T. B., Barbarese, E. &Carson, J. H. (1991) Diversification of glial lineages: a novel method to clone brain cellsin vitro on nitrocellulose substratum.Glia 4, 256–68.
Chabot, P. &Vincent, M. (1990) Transient expression of an intermediate filament-associated protein (IFAPa-400) duringin vivo andin vitro differentiation of chick embryonic cells derived from neuroectoderm.Developmental Brain Research 54, 195–204.
Chisholm, J. C. &Houliston, E. (1987) Cytokeratin filament assembly in the pre-implantation mouse embryo.Development 101, 565–82.
Chiu, F. C., Norton, W. T. &Fields, K. L. (1981) The cytoskeleton of primary astrocytes in culture contains actin, glial fibrillary acidic protein, and the fibroblast-type filament protein, vimentin.Journal of Neurochemistry 37, 147–55.
Choi, B. H. (1990) Gliogenesis in the developing human fetal brain. InDifferentiation and functions of glial cells (edited byLevi, G.) pp. 9–16. New York: Wiley-Liss.
Choi, B. H. &Kim, R. C. (1984) Expression of glial fibrillary acidic protein in immature oligodendroglia.Science 223, 407–9.
Culican, S. M., Baumrind, N. L., Yamamoto, M. &Pearlman, A. L. (1990) Cortical radial glia: Identification in tissue culture and evidence for their transformation to astrocytes.Journal of Neuroscience 10, 684–92.
Dahl, D. (1981) The vimentin-GFA protein transition in rat neuroglia cytoskeleton occurs at the time of myelination.Journal of Neuroscience Research 6, 741–8.
Dahl, D., Bignami, A., Weber, K. &Osborn, M. (1981a) Filament proteins in rat optic nerves undergoing Wallerian degeneration: localization of vimentin, the fibroblast 100-A filament protein, in normal and reactive astrocytes,Experimental Neurology 73, 496–506.
Dahl, D., Rueger, D. C., Bignami, A., Weber, K. &Osborn, M. (1981b) Vimentin, the 57,000 molecular weight protein of fibroblast filaments, is the major cytoskeletal component in immature glia.European Journal of Cell Biology 24, 191–6.
Dale, B. A., Resing, K. A. &Haydock, P. V. (1990) Filaggrins. InCellular and Molecular Biology of Intermediate Filaments (edited byGoldman, R. D. &Steinert, P. M.) pp. 393–412. New York: Plenum.
Edwards, M. A., Yamamoto, M. &Caviness, V. S., Jr. (1990) Organization of radial glia and related cells in the developing murine CNS: an analysis based on a new monoclonal antibody marker.Neuroscience 36, 121–44.
Eng, L. F., Vanderhaeghen, J. J., Bignami, A. &Gerstl, B. (1971) An acidic protein isolated from fibrous astrocytes.Brain Research 28, 351–4.
Evrard, C., Borde, I., Marin, P., Galiana, E., Premont, J., Gros, F. &Rouget, P. (1990) Immortalization of bipotential and plastic glio-neuronal precursor cells.Proceedings of the National Academy of Sciences (USA) 87, 3062–6.
Fedoroff, S. (1986) Prenatal ontogenesis of astrocytes. InAstrocytes, Vol. 1 (edited byFedoroff, S. &Vernadakis, A.) pp. 35–74. New York: Academic Press.
Fliegner, K. H. &Liem, R. K. H. (1991) Cellular and molecular biology of neuronal intermediate filaments.International Review of Cytology 131, 109–67.
Foisner, R. &Wiche, G. (1991) Intermediate filament-associated proteins.Current Opinion on Cell Biology 3, 75–81.
Frederiksen, K. &Mckay, D. G. (1988) Proliferation and differentiation of rat neuroepithelial precursor cellsin vivo.Journal of Neuroscience 8, 1144–51.
Gasser, U. E. &Hatten, M. E. (1990) Neuron-glia interactions of rat hippocampal cellsin vitro: glial-guided neuronal migration and neuronal regulation of glial differentiation.Journal of Neuroscience 10, 1276–86.
Gilmore, S. A. (1971) Neuroglial population in the spinal white matter of neonatal and early postnatal rats: an autoradiographic study of numbers of neuroglia and changes in their proliferative activity.Anatomical Record 171, 283–92.
Goldman, J. E. &Vaysse, P. J.-J. (1991) Tracing glial cell lineages in the mammalian forebrain.Glia 4, 149–56.
Goldman, J. E., Geier, S. S. &Hirano, M. (1986) Differentiation of astrocytes and oligodendrocytes from germinal matrix cells in primary culture.Journal of Neuroscience 6, 52–60.
Goldman, J. E., Schaumburg, H. H. &Norton, W. T. (1978) Isolation and characterization of glial filaments from human brain,Journal of Cell Biology 78, 426–40.
Goldman, R. D. &Steinert, P. M. (eds.) (1990)Cellular and Molecular Biology of Intermediate Filaments. New York: Plenum.
Hirano, M. &Goldman, J. E. (1988) Gliogenesis in rat spinal cord: evidence for origin of astrocytes and oligodendrocytes from radial precursors.Journal of Neuroscience Research 21, 155–67.
Hirano, S., Fuse, S. &Sohal, G. S. (1991) The effect of the floor plate on pattern and polarity in the developing central nervous system.Science 251, 310–12.
Hockfield, S. &Mckay, R. D. G. (1985) Identification of major cell classes in the developing mammalian nervous system.Journal of Neuroscience 5, 3310–28.
Houle, J. &Fedoroff, S. (1983) Temporal relationship between the appearance of vimentin and neural tube development.Developmental Brain Research 9, 189–95.
Jackson, B. W., Grund, C., Winter, S., Franke, W. W. &Illmensee, K. (1981) Formation of cytoskeletal elements during mouse embryogenesis. II. Epithelial differentiation and intermediate-sized filaments in early postimplantation embryos.Differentiation 20, 203–16.
Landry, C. F., Ivy, G. O. &Brown, I. R. (1990) Developmental expression of glial fibrillary acidic protein mRNA in the rat brain analyzed byin situ hybridization.Journal of Neuroscience Research 25, 194–203.
Lazarides, E. (1980) Intermediate filaments as mechanical integrators of cellular space.Nature 283, 249–53.
Lehtonen, E., Lehto, V.-P., Vartio, T., Badley, R. A. &Virtanen, I., (1983) Expression of cytokeratin polypeptides in mouse oocytes and preimplantation embryos.Developmental Biology 100, 158–65.
Levitt, P., Cooper, M. L. &Rakic, P. (1981) Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis.Journal of Neuroscience 1, 27–39.
Lieska, N., Yang, H.-Y. &Goldman, R. D. (1985) Purification of the 300 k intermediate filament-associated protein and itsin vitro recombination with intermediate filaments.Journal of Cell Biology 100, 802–13.
Liuzzi, F. J. &Miller, R. H. (1987) Radially oriented astrocytes in the normal adult rat spinal cord.Brain Research 403, 385–8.
Lukas, Z., Draber, P., Bucek, J., Draberova, E., Viklicky, V. &Staskova, Z. (1989) Expression of vimentin and glial fibrillary acidic protein in human developing spinal cord.Histochemical Journal 21, 693–702.
Mcdermott, K. W. G. &Lantos, P. L. (1989) The distribution of glial fibrillary acidic protein and vimentin in postnatal marmoset (Callithrix jacchus) brain.Developmental Brain Research 45, 169–77.
Misson, J.-P., Yamamoto, M., Edwards, M. A., Schwarting, V. S. &Caviness, V. S., Jr. (1987) Identification of radial glial cells within the developing murine central nervous system using a new immuno-histochemical marker.Society for Neuroscience Abstracts 13, 1465.
Misson, J.-P., Edwards, M. A., Yamamoto, M. &Caviness, V. S., Jr. (1988) Identification of radial glial cells within the developing murine central nervous system: studies based upon a new immunohistochemical marker.Developmental Brain Research 44, 95–108.
Misson, J.-P., Takahashi, T. &Caviness, V. S., Jr. (1991) Ontogeny of radial and other astroglial cells in murine cerebral cortex.Glia 4, 138–48.
Osborn, M., Ludwig, M., Weber, K., Bignami, A., Dahl, D. &Bayreuther, K. (1981) Expression of glial and vimentin type intermediate filaments in cultures derived from human glial material.Differentiation 19, 161–7.
Pixley, S. K. R. &De Vellis, J. (1984) Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin.Developmental Brain Research 15, 201–9.
Raff, M. C. (1989) Glial cell diversification in the rat optic nerve.Science 243, 1450–5.
Raju, T., Bignami, A. &Dahl, D. (1981)In vivo andin vitro differentiation of neurons and astrocytes in the rat embryo.Developmental Biology 85, 344–57.
Rakic, P. (1972) Mode of cell migration to the superficial layers of fetal monkey neocortex.Journal of Comparative Neurology 145, 61–84.
Ramon Y., Cajal, S. (1911)Histologie du Système Nerveux de l'Homme et des Vertébrés, Vol. II Paris: Maloine. (Reprinted by Madrid: Consejo Superior de Investigaciones Cientificas, Instituto Ramon y Cajal, 1955.)
Schiffer, D., Giordana, M. T., Mighili, A., Gianccone, G., Pezzotta, S. &Mauro, A., (1986) Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain.Brain Research 374, 110–18.
Schmechel, D. E. &Rakic, P. (1979) A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes.Anatomy and Embryology 156, 115–52.
Schnitzer, J. (1988) Immunocytochemical studies on the development of astrocytes, Muller (glial) cells, and oligodendrocytes in the rabbit retina.Developmental Brain Research 44, 59–72.
Schnitzer, J., Franke, W. W. &Schachner, M. (1981) Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of the developing and adult mouse nervous system.Journal of Cell Biology 90, 435–47.
Shaw, F., Osborn, M. &Weber, K. (1981) An immunofluor escence microscopical study of the neurofilament triplet proteins, vimentin and glial fibrillary acidic protein within the adult rat brain.European Journal of Cell Biology 26, 68–82.
Skoff, R. P. (1990) Gliogenesis in rat optic nerve: astrocytes are generated in a single wave before oligodendrocytes.Developmental Biology 139, 149–68.
Skoff, R. P. &Knapp, P. E. (1991) Division of astroblasts and oligodendroblasts in postnatal rodent brain: evidence for separate astrocyte and oligodendrocyte lineages.Glia 4, 165–74.
Steinert, P. M. &Roop, D. R. (1988) Molecular and cellular biology of intermediate filaments.Annual Review of Biochemistry 57, 593–625.
Valentino, K. L., Jones, E. G. &Kane, S. A. (1983) Expression of GFAP immunoreactivity during development of long fiber tracts in the rat CNS.Brain Research 285, 317–36.
Vaysse, P. J.-J. &Goldman, J. E. (1990) A clonal analysis of glial lineages in neonatal forebrain developmentin vitro.Neuron 5, 227–35.
Weinstein, D. E., Shelanski, M. L. &Liem, R. K. H. (1991) Supression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons.Journal of Cell Biology 112, 1205–13.
Yang, H.-Y., Lieska, N., Goldman, A. E. &Goldman, R. D. (1985) A 300,000-mol-wt intermediate filament-associated protein in baby hamster kidney (BHK-21) cells.Journal of Cell Biology 100, 620–31.
Yang, H.-Y., Lieska, N. &Goldman, R. D. (1990) Intermediate filament-associated proteins, inCellular and Molecular Biology of Intermediate Filaments (edited byGoldman, R. D. &Steinert, P. M.) pp. 371–91. New York: Plenum.
Yang, H.-Y., Lieska, N., Goldman, A. E. &Goldman, R. D. (1992a) Colchicine-sensitive and cholchicine-insensitive intermediate filament systems distinguished by a new intermediate filament-associated protein, IFAP-70/280 kD.Cell Motility and the Cytoskeleton 22, 185–99.
Yang, H.-Y., Lieska, N., Goldman, R. D., Johnson-Seaton, D. &Pappas, G. D. (1992b) Distinct developmental subtypes of cultured non-stellate rat astrocytes distinguished by a new glial intermediate filament-associated protein.Brain Research 573, 161–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yang, H.Y., Lieska, N., Shao, D. et al. Immunotyping of radial glia and their glial derivatives during development of the rat spinal cord. J Neurocytol 22, 558–571 (1993). https://doi.org/10.1007/BF01189043
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01189043