Conclusions
-
1.
Temperature intervals and kinetic parameters have been established for the evolution, from TiO2, of n-butylamine molecules forming surface hydrogen and coordination bonds with the adsorption centers; also, the temperature interval and kinetic parameters have been established for processes of thermolysis of surface amino compounds with a Ti-N σ-bond.
-
2.
The character of adsorptive interaction of n-butylamine with TiO2 does not depend on the degree of hydroxylation of the surface.
-
3.
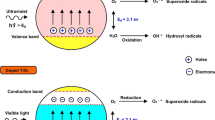
When the amine-modified surface of rutile is exposed to light at −20°C, significant quantities of products of n-butylamine oxidation and autocondensation are formed. A decrease in the degree of hydroxylation of the TiO2 surface increases the photochemical stability of the adsorption system.
Similar content being viewed by others
Literature cited
M. Primet, P. Pichat, and M.-V. Matien, J. Phys. Chem.,75, 1221 (1971).
G. D. Parfitt, J. Ramsbotham, and C. H. Rochester, J. Chem. Soc, Faraday Trans. 1,67, 841 (1971).
A. A. Isirikyan, S. S. Mikhailova, I. A. Polunina, et al., Kolloidn. Zh.,40, No. 2, 349 (1978).
M. Hermann and H. P. Boehm, Z. Anorg. Allgem. Chera.,368, No. 1, 73 (1969).
A. V. Uvarov and E. S. Pryakhina, Lakokras. Mater. Ikh Primen., No. 2, 14 (1967).
A. A. Ziganenko, V. N. Filimonov, and D. V. Pozdniakov, J. Mol. Struct.,29, 299 (1975).
J. Graham, C. J. Rochester, and R. Rudham, J. Chem. Soc, Faraday Trans. 1,79, 2991 (1983).
A. P. Gai, G. A. Gerasimova, V. V. Gubin, L. T. Zhuravlev, S. A. Kiselev, L. N. Nikitin, and N. P. Sokolova, Summaries of Papers from All-Union Conference on Automation of Analysis of Chemical Composition of Substances [in Russian], Moscow (1980). p. 59.
E. S. Freeman and B. Carrol, J. Phys. Chem.,62, 394 (1958).
A. A. Isirikyan, in: Adsorption and Porosity [in Russian], Nauka, Moscow (1976), p. 89.
V. A. Terent'ev, Thermodynamics of the Donor-Acceptor Bond [in Russian], Izd. Saratov University (1981), p. 277.
A. G. Goroshchenko, The Chemistry of Titanium [in Russian], Part I, Naukova Dumka, Kiev (1970), p. 154.
V. Karnozhitskii, Usp. Khim.,46, No. 2, 239 (1977).
Z. N. Yaremenko and L. N. Krakhaleva, Problems in the Chemistry and Chemical Technology of Titanium Dioxide and Iron-Containing Pigments [in Russian], Moscow (1983), p, 9.
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 7, pp. 1463–1468, July, 1987.
Rights and permissions
About this article
Cite this article
Polunina, I.A., Ovchinnikova, N.S., Mikhailova, S.S. et al. Mass spectrometric study of thermodesorption of n-butylamine from titanium dioxide surface. Russ Chem Bull 36, 1350–1355 (1987). https://doi.org/10.1007/BF01557498
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01557498