Conclusions
-
1.
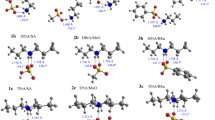
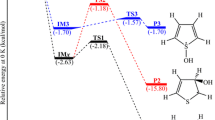
From MNDO quantum chemical calculations, opening of the episulfonium ion ring by neutral nucleophiles X (X=NH3 and HCN) and related SN2 reactions of protonated methylthiol (PMT) with X proceed through formation of pre-reaction complexes in which X is coordinated either at the reacting C atom or (only in opening of episulfonium ion rings) at the center of the C-C bond.
-
2.
In their electronic structure, the transition states for the reactions are reminiscent of a carbocation simultaneously reacting with the attacking and the leaving nucleophilic fragments (X ⋯\(\mathop C\limits^ + \) ⋯S).
-
3.
Opening of episulfonium ion rings proceeds slightly more easily (Ea ∿ 10–12 kcal/mole) than substitution in PMT (Ea ∿ 22–25 kcal/mole). The ease of ring opening for episulfonium ions is due to the large exothermicity of the reaction and the lower internal activation barrier compared with SN2 reactions in PMT.
Similar content being viewed by others
Literature cited
W. A. Smit, M. Z. Krimer, and E. A. Vorobieva, Tetrahedron Lett., 2451 (1975).
N. S. Zefirov, N. K. Sadovaya, A. M. Materremov, and I. V. Bodrikov, Zh. Org. Khim.,13, 903 (1976).
W. A. Smit, N. S. Zefirov, I. V. Bodrikov, and M. Z. Krimer, Acc. Chem. Res., 282 (1979).
D. Barton and W. D. Ollis, eds., Comprehensive Organic Chemistry, Vol. 2, Pergamon Press, New York (1979).
B. Badet, M. Julia, and M. Ramirez-Munos, Synthesis, 926 (1980).
K. Raghavachari, J. Chandrasekhar, and R. C. Burnier, J. Am. Chem. Soc.,106, 3124 (1984).
M. V. Bazilevskii, S. G. Koldobskii, and V. A. Tikhomirov, Usp. Khim.,55, 1667 (1986).
M. J. S. Dewar and W. Thiel, J. Am. Chem. Soc.,99, 4899 (1977).
M. J. S. Dewar and M. L. McKee, J. Comp. Chem.,4, 84 (1983).
M. J. S. Dewar and Ch. H. Reynolds, J. Comp. Chem.,7, 140 (1986).
G. Klopman (ed.), Chemical Reactivity and Reaction Paths, Wiley, New York (1974).
J. W. McIver, Jr. and A. Komornicki, J. Am. Chem. Soc.,94, 2625 (1972).
G. S. Hammond, J. Am. Chem. Soc.,77, 334 (1955).
A. C. Knipe, in: The Chemistry of the Sulphonium Group, Pt. 1 (C. J. M. Stirling and S. Patai, eds.), Wiley, New York (1981).
M. Meot-Ner, P. Hamlet, E. P. Hunter, and F. H. Field, J. Am. Chem. Soc.,102, 6392 (1980).
P. Kebarle, Ann. Rev. Phys. Chem.,28, 445 (1977).
J. W. Albert, Ann. Rev. Phys. Chem.,31, 2706 (1980).
G. P. Ford and C. T. Smith J. Am. Chem. Soc.,109, 1325 (1987).
J. Hayami, N. Tanaka, N. Hihara, and A. Kaji, Tetrahedron Lett., 385 (1973).
J. Chandrasekhar, S. F. Smith, and W. L. Jorgensen, J. Am. Chem. Soc.,106, 3049 (1984).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 7, pp. 1573–1580, July, 1989.
Rights and permissions
About this article
Cite this article
Faustov, V.I., Smit, W.A. Quantum chemical study of reactions of episulfonium ions. 1. Comparative MNDO study of opening of the episulfonium ion ring by neutral nucleophiles and SN2 substitution in protonated methylthiol. Russ Chem Bull 38, 1439–1445 (1989). https://doi.org/10.1007/BF00978435
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00978435