Summary
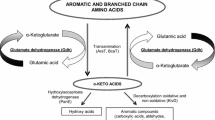
Cell-free extracts of Leuconostoc and Lactococcus species were tested for their α-acetolactate synthase and α-acetolactate decarboxylase activities. In Leuconostoc mesenteroides subsp. cremoris, Leuconostoc mesenteroides subsp. mesenteroides and Leuconostoc lactis, the Km of α-acetolactate synthase for pyruvate was close to 10 mM whereas it was 30 mM in Lactococcus lactis subsp. lactis biovar. diacetylactis. The Km of α-acetolactate decarboxylase for α-acetolactic acid was very low (0.3 mM) in Leuconostoc species in comparison to Lactococcus lactis subsp. lactis biovar. diacetylactis (60 mM). In the latter bacterium, α-acetolactate decarboxylase showed a sigmoidal dependance upon α-acetolactic acid and was activated by the three branchedchain amino acids: leucine, isoleucine and valine.
Similar content being viewed by others
References
Chopin, A. (1993) FEMS Microbiol. Rev. 12, 21–38.
Cogan, T.M. (1975) J. Dairy Res. 42, 139–146.
Cogan, T.M. (1982) Ir. J. Fd Sci. Technol. 6, 69–78.
Cogan, T.M. (1987) J. Appl. Bacteriol. 63, 551–558.
Cogan, T.M., Fitzgerald, R.J. and Doonan, S. (1984) J. Dairy Res. 51, 597–604.
de Man, J.C., Rogosa, M. and Sharpe, M.E. (1960) J. Appl. Bacteriol. 23, 130–135.
Hugenholz, J. (1993) FEMS Microbiol. Rev. 12, 165–178.
Hugenholtz, J. and Starrenburg, M.J.C. (1992) Appl. Microbiol. Biotechnol. 38, 17–22.
Lin, J., Schmitt, P. and Diviès, C. (1991) Appl. Microbiol. Biotechnol. 34, 628–631.
Lowry, O.H., Rosenbrough, N.J., Farr, A.G. and Randall, R.J. (1951) J. Biol. Chem. 193, 265–275.
Rasmussen, A.M., Gibson, R.M., Godtfredsen, S.E. and Ottesen, M. (1985) Carlsberg Res. Commun. 50, 73–82.
Schmitt, P., Couvreur, C., Cavin, J.F., Prévost, H. and Diviès, C. (1988) Appl. Microbiol. Biotechnol. 29, 430–436.
Schmitt, P. and Diviès, C. (1992) Appl. Microbiol. Biotechnol. 37, 426–430.
Schmitt, P., Diviès, C. and Cardona, R. (1992) Appl. Microbiol. Biotechnol. 36, 679–683.
Snoep, J.L., Teixeira de Mattos, M.J., Starrenburg, M.J.C. and Hugenholtz, J. (1992) J. Bacteriol. 174, 4838–4831.
Starrenburg, M.J.C. and Hugenholtz, J. (1991) Appl. Environ. Microbiol. 57, 3535–3540.
Terzaghi, B.E. and Sandine, W.E. (1975) Appl. Microbiol. 29, 807–813.
Westerfeld, W.W. (1945) J. Biol. Chem. 16, 495–502.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Monnet, C., Phalip, V., Schmitt, P. et al. Comparison of α-acetolactate synthase and α-acetolactate decarboxylase in Lactococcus spp. and Leuconostoc spp.. Biotechnol Lett 16, 257–262 (1994). https://doi.org/10.1007/BF00134622
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00134622