Summary
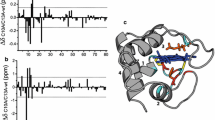
The hydrogen-deuterium exchange rates of the reduced and oxidized forms ofRhodobacter' capsulatus cytochrome c2 were studied by1H−15N homonuclear multiple quantum correlation spectroscopy. Minimal differences were observed for the N- and C-terminal helices on changing redox state suggesting that although these helices are structurally important they do not affect the relative stability of the two redox states and hence may not be important in determining the redox potential differences observed amongst the class I c-type cytochromes. However, significant differences were observed for other regions of the protein. For example, all slow exchanging protons of the helix spanning Phe82 to Asp87 are similarly affected on reduction indicating that the unfolding equilibrium of this helix is altered between the two redox states. Other regions are not as simple to interpret; however, the difference in NH exchange rates between the redox states for a number of residues including His17, Leu37, Arg43, Ala45, Gly46, Ile57, Val58, Leu60, Gly61 and Leu100 suggest that interactions affecting the causes of these differences may be important factors in determining redox potential.
Similar content being viewed by others
Abbreviations
- NMR:
-
nuclear magnetic resonance
- HMQC:
-
homonuclear multiple quantum correlation
- NOESY:
-
nuclear Overhauser effect spectroscopy
References
Bax, A., Griffey, R.H. and Hawkins, B.L. (1983)J. Magn. Reson.,55, 301–315.
Bhatia, G.E., (1981) Ph.D. Thesis, University of California, San Diego.
Bushnell, G.W., Louie, G.V. and Brayer, G.D. (1990)J. Mol. Biol.,214, 585–595.
Caffrey, M.S. (1990) Ph.D. Thesis, University of Arizona, Tucson.
Cusanovich, M.A., Meyer, T.E. and Tollin, G. (1988) InAdvances in Inorganic Biochemistry, Heme Proteins 7, (Eds, Eichhorn, G.L. and Manzilli, L. G.) Elsevier, New York, pp. 37–92.
Dickerson, R.E. and Timkovich, R. (1975) inThe Enzymes, 3rd ed., Vol. 11 (Ed, Boyer, P.D.), Academic Press, N.Y., pp. 397–547.
Englander, J.J., Rogero, J.R. and Englander, S.W. (1983)J. Mol. Biol.,169, 325–344.
Englander, S.W. and Kallenbach, N.R. (1984)Q. Rev. Biophys.,16, 521–655.
Gooley, P.R., Caffrey, M.S., Cusanovich, M.A. and MacKenzie, N.E. (1990)Biochemistry,29, 2278–2290.
Gooley, P.R. and MacKenzie, N.E. (1990)FEBS Lett.,260, 225–228.
Gooley, P.R., Caffrey, M.S., Cusanovich, M.A. and MacKenzie, N.E. (1991)Eur. J. Biochem.,196, 653–661.
Hvidt, A. and Nielsen, S.O. (1966)Adv. Protein Chem.,21, 287–386.
Jeener, J., Meier, B.H., Bachman, P. and Ernst, R.R. (1979)J. Chem. Phys.,71, 4546–4553.
Kassner, R.J. (1972)Proc. Natl. Acad. Sci. USA,69, 2263–2267.
Kuwajima, K. and Baldwin, R.L. (1983)J. Mol. Biol. 169, 299–323.
Louie, G.V. and Brayer, G.D. (1990)J. Mol. Biol.,214, 527–555.
Molday, R.S., Englander, S.W. and Kallen, R.G. (1972)Biochemistry,11, 150–158.
Roder, H., Wagner, G. and Wüthrich, K. (1985)Biochemistry,24, 7396–7407.
Takano, T. and Dickerson, R.E. (1981a)J. Mol. Biol.,153, 79–94.
Takano, T. and Dickerson, R.E. (1981b)J. Mol. Biol.,153, 95–115.
Wagner, G. and Wüthrich, K. (1982)J. Mol. Biol.,160, 343–361.
Wand, A.J., Roder, H. and Englander, S.W. (1986)Biochemistry,25, 1107–1114.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gooley, P.R., Zhao, D. & MacKenzie, N.E. Comparison of amide proton exchange in reduced and oxidizedRhodobacter capsulatus cytochrome c2: A1H−15N NMR study. J Biomol NMR 1, 145–154 (1991). https://doi.org/10.1007/BF01877226
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01877226