Abstract
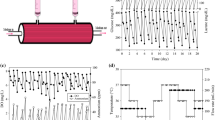
In the study presented here a laboratory scale (150 ml) fluidized bed bioreactor was used as a tool for making kinetic measurements on the production of monoclonal antibodies (MAb) with a hybridoma cell line. We determined the influence of dissolved oxygen tension (DOT) on the metabolic activity of a hybridoma population immobilized in macroporous collagen microspheres. The data obtained showed a reduction of the metabolic activity of the immobilized population at reduced DOT, the total number of immobilized cells, however, remained constant. At decreasing DOT an increasing lactate yield from glucose at reduced glutamine consumption was noticed, indicating a shift in the pattern of substrate usage. A mathematical description of maintenance metabolism was formulated and the parameters of growth and maintenance requirements were calculated. A growth associated MAb production was determined under the conditions applied leading to space time yields of 225 mg MAb per liter of total reactor volume and day.
Similar content being viewed by others
References
Almgren J, Nilsson C, Petersson AC and Nilsson, K (1991) Cultispher—macroporous gelatin microcarrier—new applications. In: Spier RE, Griffiths JB and Meignier B (eds) Production of Biologicals from Animal Cells in Culture (pp. 434–438) Oxford, Butterworth-Heinemann.
Altshuler GL, Dziewulski DM, Sowek JA and Belfort G (1986) Continuous hybridoma growth and monoclonal antibody production in hollow fiber reactors-separators. Biotechnol. Bioeng. 28: 646–658.
Bergmeyer HU (ed) (1985) Methods of Enzymatic Analysis 8, VCH Verlagsgesellschaft Weinheim a) 357–363; b) 454–461.
Blüml G, Reiter M, Zach N, Gaida T, Schmatz C, Strutzenberger C, Mohr T and Katinger H (1992) Development of a new type of macroporous carrier. In: Spier RE, Griffiths JB and MacDonald C (eds) Animal Cell Technology: Development, Processes and Products (pp. 501–504), Oxford, Butterworth-Heinemann.
Boraston R, Thompson PW, Garland S and Birch JR (1984) Growth and oxygen requirements of antibody producing mouse hybridoma in suspension culture. Develop. Biol. Standard 55: 103–111.
Cadic Ch, Dupuy B, Pianet I, Merle M, Margerin Ch and Bezian JH (1992) In vitro culture of hybridoma cells in agarose beads producing antibody secretion for two weeks. Biotechnol. Bioeng. 39: 108–112.
Cooper PD, Burt AM and Wilson JN (1958) Critical effect of oxygen tension on rate of growth of animal cells in continuous suspension culture. Nature 182: 1508–1509.
Flickinger M. (November 13–15, 1991) Proceedings of the 4th Annual Meeting of the Japanese Association for Animal Cell Technology. Fukukoka, Japan, to be published.
Frame KK and Hu WS (1991) Kinetic study of hybridoma cell growth in continuous culture: 1. A model for nonproducing cells. Biotechnol. Bioeng. 37: 55–64.
Glacken MW, Fleischaker E and Sinskey AJ (1986) Reduction of waste product excretion via nutrient control: Possible strategies for maximising product and cell yields on serum in cultures of mammalian cells. Biotechnol. Bioeng. 28: 1376–1389.
Hambach B, Biselli M, Runstadler PW and Wandrey C (1992) Development of a reactor integrated aeration system for cultivation of animal cells in fluidized beds. In: Spier RE, Griffiths JB and MacDonald C (eds) Animal Cell Technology: Development, Processes and Products (pp. 381–384). Oxford, Butterworth & Heinemann.
Handa-Corrigan A, Nikolay S and Spier RE (1991) Biochemical control of monoclonal antibody secretion in hollow fibre bioreactors. In: Spier RE, Griffiths JB and Meignier B (eds) Production of Biologicals from Animal Cells in Culture (pp. 489–494), Oxford, Butterworth & Heinemann.
Hayman EG, Ray NG and Runstadler PW (1988) In: Moody DU and Baker PE (eds) Bioreactors and Biotransformations (pp. 99–110), London and New York, Elsevier.
Hiller GW, Aeschlimann AD, Clark DS and Blanch HW (1991) A kinetic analysis of hybridoma growth and metabolism in continuous suspension culture on serum-free medium. Biotechnol. Bioeng. 38: 733–741.
Keller J, Dunn IJ and Heinzle J (1991) Improved performance of the fluidized bed reactor for the cultivation of animal cells. In: Spier, RE, Griffiths JB and Meignier B (eds) Production of Biologicals from Animal Cells in Culture (pp. 513–515), Oxford, Butterworth-Heinemann.
Kratje RB and Wagner R (1992) Evaluation of production of recombinant human interleukin-2 in fluidized bed bioreactor. Biotechnol. Bioeng. 39: 233–242.
Lazar A, Reuveny S, Mizrahi A, Avtalion M and Whiteside JP (1987) Production of biologicals by animal cells immobilized in a polyurethane foam matrix. In: Spier RE and Griffiths JB (eds) Modern Approaches to Animal Cell Technology (pp. 437–448), Guildford, Butterworth.
Lee GM and Palsson BO (1990) Immobilization can improve the stability of hybridoma antibody productivity in serum-free media. Biotechnol. Bioeng. 36: 1049–1055.
Lehmann J, Vorlop J and Büntemeyer H (1988) Bubble-free reactors and their development for continuous cell recycle. In: Spier RE, Griffiths JB (eds) Animal Cell Biotechnology, Vol 3 (pp. 222–237), London, Academic Press.
Looby D and Griffiths JB (1988) Fixed bed porous glass sphere (porosphere) bioreactors for animal cells, Cytotechnology 1: 339–346.
Lüllau E, Dreisbach C, Grogg AF, Biselli M and Wandrey C (1992) Immobilization of animal cells on chemically modified siran carriers. In: Spier RE, Griffiths JB and MacDonald C (eds) Animal Cell Technology: Development, Processes and Products (pp. 469–475), Oxford, Butterworth-Heinemann.
Marc A, Wagner A, Martial A, Goergen JL, Engasser JM, Geaugey V and Pinton H (1991) Potential and pitfalls of using LDH release for the evaluation of animal cell death kinetics. In: Spier RE, Griffiths JB and Meignier B (eds) Production of Biologicals from Animal Cells in Culture (pp. 569–574), Oxford, Butterworth-Heinemann.
Meilhoc E., Wittrup KD and Bailey JE (1990) Influence of dissolved oxygen concentration on growth, mitochondrial function and antibody production of hybridoma cells in batch culture. Bioproc. Eng. 5: 263–274.
Miller WM, Blanch HW and Wike CR (1988a) A kinetic analysis of hybridoma growth and metabolism in batch and continuous suspension culture: Effect of nutrient concentration, dilution rate and pH. Biotechnol. Bioeng. 32: 947–965.
Miller WM, Wilke CR and Blanch HW (1988b) Transient responses of hybridoma metabolism to changes in the oxygen supply rate in continuous culture. Bioproc. Eng. 3: 103–111.
Ozturk SS and Palsson BO (1990) Effect of dissolved oxygen on hybridoma cell growth metabolism and antibody production kinetics in continuous culture. Biotechnol. Progr. 6: 437–446.
Ozturk SS and Palsson BO (1991a) Growth, metabolic and antibody production kinetics of hybridoma cell culture: 1. Analysis of data from controlled batch reactors. Biotechnol. Progr. 7: 471–480.
Ozturk SS and Palsson BO (1991b) Growth, metabolic and antibody production kinetics of hybridoma cell culture: 2. Effect of serum concentration, dissolved oxygen concentration and medium pH in a batch reactor. Biotechnol. Progr. 7: 481–494.
Phillips HA, Scharer JM, Bols NC and Moo-Young M (1987) Effect of oxygen on antibody productivity in hybridoma culture. Biotechnol. Lett. 9(11): 745–750.
Pirt SJ (1965) The maintenance energy of bacteria in growing cultures. Proc. Royal. Soc. 163: 224–231.
Ray NG, Karkare SB and Runstadler PW (1989) Cultivation of hybridoma cells in continuous culture: Kinetics of growth and product formation. Biotechnol. Bioeng. 33: 724–730.
Reuveny S, Velez D, Miller L and Macmillan JD (1986) Comparison of cell propagation methods for their effect on monoclonal antibody yield in fermentors. J. Immunol. Meth. 86: 61–69.
Runstadler PW and Cernek R (1988) Large-scale fluidized-bed, immobilized cultivation of animal cells at high densities. In: Spier RE and Griffiths JB (eds) Animal Cell Biotechnology Vol. 3 (pp. 306–320), London, Academic Press.
Runstadler PW, Ozturk SS and Ray NG. An evaluation of hybridoma cell specific productivity: Perfused immobilized, continuous suspension and batch suspension cultures, Proceedings of the 4th Annual Meeting of the Japanese Association for Animal Cell Technology. Fukukoka, Japan (1991), Kluwer Academic Publishers, Dordrecht, The Netherlands, to be published.
Wilson DW, Erecinska M, Drown C and Silver IA (1979) The oxygen dependency of cellular energy metabolism. Arch. Biochem. Biophys. 195(2): 485–493.
Zielke HR, Zielke CL and Ozand PT (1984) Glutamine: A major energy source for cultured mammalian cells. Fed. Proc. 43: 121–125.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thömmes, J., Gätgens, J., Biselli, M. et al. The influence of dissolved oxygen tension on the metabolic activity of an immobilized hybridoma population. Cytotechnology 13, 29–39 (1993). https://doi.org/10.1007/BF00749973
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00749973