Abstract
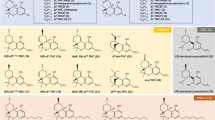
A cannabinoid pseudoreceptor model for the CB1-receptor has been constructed for 31 cannabinoids using the molecular modelling software YAK. Additionally, two CoMFA studies were performed on these ligands, the first of which was conducted prior to the building of the pseudoreceptor. Its pharmacophore is identical with the initial superposition of ligands used for pseudoreceptor construction. In contrast, the ligand alignment for the second CoMFA study was taken directly from the final cannabinoid pseudoreceptor model. This altered alignment gives markedly improved cross-validated r2 values as compared to those obtained from the original alignment with\({\text{r}}_{{\text{cross}}}^2 \) values of 0.79 and 0.63, respectively, for five components. However, the pharmacophore alignment has the better predictive ability. Both the CoMFA and pseudoreceptor methods predict the free energy of binding of test ligands well.
Similar content being viewed by others
References
Gaoni, Y. and Mechoulam, R., J. Am. Chem. Soc., 93 (1971) 217.
Razdan, R.K., Pharmacol. Rev., 38 (1986) 75.
Kovar, K.-A., Pharm. Unserer Zeit, 3 (1981) 65.
Kovar, K.-A., Dtsch. Apoth. Ztg., 43 (1992) 2302.
Schmidbauer, W. and Scheidt, J., Handbuch der Rauschdrogen, 6th ed., Nymphenburger Verlag, München, Germany, 1981.
Mechoulam, R. and Lander, N., Pharm. Int., 1 (1980) 19.
Compton, D.R., Rice, K.C., DeCosta, B.R., Razdan, R.K., Melvin, L.S., Johnson, M.R. and Martin, B.R., J. Pharmacol. Exp. Ther., 265 (1993) 218.
Martin, W.J., Patrick, S.L., Coffin, P.O., Tsou, K. and Walker, J.M., Life Sci., 56 (1995) 2103.
Johnson, M.R., Melvin, L.S., Althuis, T.H., Bindra, J.S., Harbert, C.A., Milne, G.M. and Weissman, A., J. Clin. Pharmacol., 21 (1981) 271.
Johnson, M.R. and Melvin, L.S., In Mechoulam, R. (Ed.) Cannabinoids as Therapeutic Agents, CRC Press, Boca Raton, FL, U.S.A., 1986, pp. 121–146.
Reggio, P.H., Seltzman, H.H., Compton, D.R., Prescott, W.R., Martin, J.R. and Martin, B.R., Mol. Pharmacol., 38 (1990) 854.
Little, P.J., Compton, D.R., Mechoulam, R. and Martin, B.R., Biochem. Behav., 32 (1989) 661.
Thomas, B.F., Compton, D.R., Martin, B.R. and Semus, S.F., Mol. Pharmacol., 40 (1991) 656.
Howlett, A.C., Johnson, M.R., Melvin, L.S. and Milne, G.M., Mol. Pharmacol., 33 (1988) 297.
Reggio, P.H., Panu, A.M. and Miles, S., J. Med. Chem., 36 (1993) 1761.
Makriyannis, A. and Rapaka, R.S., Life Sci., 47 (1990) 2173.
Devan, W.A., Dysarz, F.A., Johnson, M.R., Melvin, L.S. and Howlett, A.C., Mol. Pharmacol., 34 (1988) 605.
Matsuda, L.A., Lolait, S.J., Brownstein, M.J., Young, A.C. and Bonner, T.J., Nature, 346 (1990) 561.
Sean, M., Kerrie, L.T. and Muna, A.-S., Nature, 365 (1993) 61.
Compton, D.R., Johnson, M.R., Melvin, L.S. and Martin, B.R., J. Pharmacol. Exp. Ther., 260 (1992) 201.
Cambridge Crystallographic Database, Crystallographic Data Centre, University Chemical Laboratory, Cambridge, U.K.
Stewart, J.J.P., MOPAC 5.0. A general molecular orbital package (QCPE 455), Frank J. Seiler Research Laboratory, Colorado Springs, CO, U.S.A.
Briem, H., Dissertation, Berlin, Germany, 1990.
Archer, R.A., Boyd, D.B., Demarco, P.V., Tyminski, L.J. and Allinger, N.L., J. Am. Chem. Soc., 92 (1970) 5200.
Reggio, P.H. and Mazurek, A.P., J. Mol. Struct. (THEOCHEM), 149 (1987) 331.
Kriwacki, R.W. and Makriyannis, A., Mol. Pharmacol., 35 (1989) 495.
Sufrin, J.R., Dunn, D.A. and Marshall, G.R., Mol. Pharmacol., 19 (1980) 307.
Marshall, G.R., Barry, C.D., Bosshard, H.E., Dammkoehler, R.A. and Dunn, D.A., Comput. Assisted Drug Design, 112 (1979) 205.
Marshall, G.R. and Motoc, J., In Burgen, A.S.V., Roberts, G.C.K. and Tute, M.S. (Eds.) Molecular Graphics and Drug Design, Elsevier, Amsterdam, The Netherlands, 1986, pp. 115–156.
Marshall, G.R. and Cramer III, R.D., Trends Pharmacol. Sci., 9 (1988) 285.
Hibert, M.F., Gittos, M.W., Middlemiss, D.N., Mir, A.K. and Fozard, J.R., J. Med. Chem., 31 (1988) 1087.
Vedani, A., Zbinden, P., Snyder, J.P. and Greenidge, P.A., J. Am. Chem. Soc., 117 (1995) 4987.
Still, W.C., Tempczyk, A., Hawley, R.C. and Hendrickson, T.J., J. Am. Chem. Soc., 112 (1990) 6127.
Thibaut, U., Folkers, G., Klebe, G., Kubinyi, H., Merz, A. and Rognan, D., In Kubinyi, H. (Ed.) 3D QSAR in Drug Design: Theory, Methods and Applications, ESCOM, Leiden, The Netherlands, 1993, pp. 711–716.
Rosenqvist, E. and Otterson, T., Acta Chem. Scand., B29 (1975) 379.
Tollenaere, J.P., Moereels, H. and Raymaekers, L.A., Atlas of the Three-dimensional Structure of Drugs, Elsevier, Amsterdam, The Netherlands, 1979, p. 232.
Binder, M., Edery, H. and Porath, G., In Nahas, G.G. and Paton, W.D.M. (Eds.) Marihuana Biological Effects, Analysis, Metabolism, Cellular Responses, Reproduction and Brain, Pergamon, Oxford, U.K., 1979, pp. 71–80.
Xie, X.-Q., Yang, D.-P., Melvin, L.S. and Makriyannis, A., J. Med. Chem., 37 (1994) 1418.
Thomas, B.F., Compton, D.R., Martin, B.R. and Semus, S.F., Mol. Pharmacol., 40 (1991) 656.
Semus, S.F., Med. Chem. Res., 1 (1992) 454.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmetzer, S., Greenidge, P., Kovar, KA. et al. Structure–activity relationships of cannabinoids: A joint CoMFA and pseudoreceptor modelling study. J Comput Aided Mol Des 11, 278–292 (1997). https://doi.org/10.1023/A:1007960712989
Issue Date:
DOI: https://doi.org/10.1023/A:1007960712989