Abstract
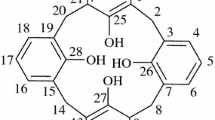
The title compounds crystallize such that bilayers of calixarenes are separated by hydrophilic layers. In each case the transition metal has, in addition to a primary sphere of ligands, a second-sphere coordination by a calixarene. [(H2O)5Ni(NC5H5)]2(Na)[calix[4]arene sulfonate]·3.5 H2O,1, crystallizes in the triclinic space groupP \(\bar 1\) witha = 12.487(4),b = 14.281(2),c = 15.055(5) Å, α = 85.66(2), β = 80.07(2), γ = 80.48(2)°, andD c = 1.64 g cm−3 forZ = 2. Refinement based on 2441 observed reflections led to a finalR value of 0.066. There are two different environments for the nickel-containing cations: one is positioned within the hydrophilic layer with the pyridine ligand intercalated into the hydrophobic calixarene bilayer and the other is also positioned within the hydrophilic layer, but the pyridine ligand is inserted into the hydrophobic cavity of the calix[4]arene. [(H2O)4Cu(NC5HS)2](H3O)3[calix[4]arene sulfonate]·10 H2O,2, crystallizes in the triclinic space groupP \(\bar 1\) witha = 15.438(4),b = 15.727(6),c = 12.121(9) Å, α = 112.74(4), β = 102.02(4), γ = 85.34(4)°, andD c = 1.57 g cm−3 forZ = 2. Refinement based on 3925 observed reflections led to a finalR value of 0.107. The structure is similar to that of 1 except that the one copper-containing cation spans the hydrophilic layer and is intercalated into the bilayer of calixarenes on one side and positioned into the calixarene cavity on the other.
Similar content being viewed by others
References
H. M. Colquhoun, J. F.Stoddart, and D. J. Williams:Angew. Chem. Int. Ed. Engl. 27, 1986 (1988).
H. M. Colquhoun, S. M. Doughty, A. M. Z. Slawin, J. F. Stoddart, and D. J. Williams:Angew. Chem. Int. Ed. Engl. 24, 134 (1985).
D. R. Alston, A. M. Z. Slawin, J. F. Stoddart, and D. J. Williams:Angew. Chem. Int. Ed. Engl. 24, 786 (1985).
T. Hoh, A. Harada, and S. Takahashi:Mem. Inst. Sci. Ind. Res., Osaka Univ. 46, 37 (1989).
D. R. Alston, A. M. Z. Slawin, J. F. Stoddart, D. J. Williams, and R. Zarzycki:Angew. Chem. Int. Ed. Engl. 27, 1184 (1988).
D. R. Alston, T. H. Lilley, J. F. Stoddart:J. Chem. Soc., Chem. Commun. 1600 (l985).
N. Kobayashi, and M. Opallo:J. Chem. Soc., Chem. Commun. 477 (l990).
B. Klingert and G. Ribs:Organometallics 9, 1135 (1990).
A. Harada, Y. Hu, S. Yamamoto, and S. Takahashi:J. Chem. Soc., Dalton Trans. 729 (1988).
P. R. Ashton, J. F. Stoddart, and R. Zarzycki:Tetrahedron Lett. 29, 2103 (1988).
J. L. Atwood, G. W. Orr, F. Hamada, R. L. Vincent, S. G. Bott, and K. D. Robinson:J. Am. Chem. Soc. 113, 2760 (1991).
S. Shinkai, K. Araki, T. Tsubaki, T. Arimura, and O. Manabe:J. Chem. Soc., Perkin Trans. I 2297 (l987).
M. R. Rosenthal and R. S. Drago:Inorg. Chem. 4, 840 (1965).
J. L. Atwood, S. G. Bott, and R. L. Vincent:J. Crystallogr. Spectrosc. Res. 20, 631 (l990).
A. W. Coleman, S. G. Bott, S. D. Morley, C. M. Means, K. D. Robinson, H. Zhang, and J. L. Atwood:Angew. Chem. Int. Ed. Engl. 27, 1361 (1988).
J. Holton, M. F. Lappert, D. G. H. Ballard, R. Pearce, J. L. Atwood, and W. E. Hunter:J. Chem. Soc., Dalton Trans. 45 (l979).
J. L. Atwood, F. Hamada, K. D. Robinson, G. W. Orr, and R. L. Vincent:Nature 349, 683 (l991).
G. Arena, R. Cali, G. G. Lombardo, E. Rizzarelli, D. Sciotto, R. Ungaro, and A. Casnati: personal communication.
J. L. Atwood, G. W. Orr, N. C. Means, F. Hamada, H. Zhang, S. G. Bott, and K. D. Robinson:Inorg. Chem. 31, 603 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Atwood, J.L., Orr, G.W., Hamada, F. et al. Calixarenes as second-sphere ligands for transition metal ions. Synthesis and crystal structure of [(H2O)5 Ni(NC5H5)]2(Na)[calix[4]arene sulfonate] · 3.5 H2O and [(H2O)4Cu(NC5H5)2]-(H3O)3 [calix[4]arene sulfonate] · 10 H2O. J Incl Phenom Macrocycl Chem 14, 37–46 (1992). https://doi.org/10.1007/BF01041364
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01041364