Abstract
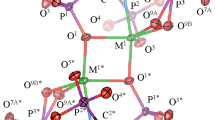
(trienH2)[CoCl4], which contains tetrahedral chlorocobaltate(II) anions, decomposes under argon in two stages via a stepwise deprotonation of the cation. The decomposition starts at 310°C with the liberation of HCl, followed at 400°C by the simultaneous release of a further mole of HCl and triene and/or its cracking products. The second decomposition stage is strongly influenced by the atmosphere. In the lower temperature region (<400°C), increasing oxygen contents of the carrier gas lead to decreasing mass losses. Therefore, the solid residues contain various amounts of C,N-containing products as well as coke. The thermal decomposition of the iron(III) compound, which contains μ-oxalato-bridged FeCl4 units, begins with the dehydratation followed by the decay of the complex anion to produce CO, CO2, and HCl. Instead of a binuclear, monooxobridged chloroferrate(III) complex, a [FeCl4]− — containing compound is proposed as one of the final products. The third decomposition stage, partially overlaying the preceding one, is the degradation of the organic cation as found for the cobalt compound. The results of thein situ-TA-MS measurements are compared with those obtained from usual TA techniques as well as from the residue characterization by X-ray diffraction, Raman spectroscopy, and chemical analysis.
Similar content being viewed by others
References
M. Feist, S. Trojanov and E. Kemnitz, Z. Naturforsch., 51b (1996) 1137.
M. Feist, S. Trojanov and E. Kemnitz, Z. Anorg. Allg. Chem., 621 (1995) 1775.
M. Feist, S. Trojanov and E. Kemnitz, Z. Naturforsch., 51b (1996) 9.
M. Feist, S. Trojanov and E. Kemnitz, Inorg. Chem., 35 (1996) 3067.
A. Heß, and E. Kemnitz, J. Catal., 149 (1994) 449.
E. Kemnitz and K.-U. Niedersen, J. Catal., 155 (1995) 283.
Heyong He, K. Alberti, T. L. Barr and J. Klinowski, J. Phys. Chem., 97 (1993) 13703.
G. Lombardi, For Better Thermal Analysis, Special Edn. of the Intern. Confederation for Thermal Analysis, University of Rome, 1980.
F. W. McLafferty and F. Turecek: Interpretation von Massenspektren, Spectrum Akademischer Verlag, Heidelberg, Berlin, Oxford 1995.
D.-H. Menz, W. Wilde and L. Kolditz, J. Fluorine Chem., 24 (1984) 345.
K. Heide, D.-H. Menz, C. Schmidt and L. Kolditz, Z. Anorg. Allg. Chem., 520 (1985) 32.
National Institute of Standards and Technology (NIST), USA, MS-Data Collection.
T. Hirata, T. Kashigawi and J. E. Brown, Macromolecules, 18 (1985) 1410.
R. Kunze and D. Neubert: TG-MS-Untersuchungen zum thermischen Abbau von Polymeren, Frühjahrstagung Polymerphysik Marburg, in: Verhandl. Dt. Phys. Ges., H.1, 1996, S.24.
M. G. B. Drew, V. McKee and S. M. Nelson, J. Chem. Soc. Dalton Trans., (1980) 80.
J. Shamir, B. J. Van der Keeken, M. A. Herman and R. Rafaeloff, J. Raman Spectr., 11 (1981) 215.
J. S. Avery, C. D. Burbridge and D. M. L. Goodgame, Spectrochim. Acta, 24A (1968) 1721.
K. Witke, K.-D. Schleinitz, M. Feist, D. Hass and M. Lorenz, Z. Anorg. Allg. Chem., 551 (1987) 215.
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley & Sons, New York, Chichester, 3rd edn. 1978.
R. J. Thibeau, C. W. Brown and R. H. Heidersbach, Appl. Spectr., 32 (1978) 532.
Author information
Authors and Affiliations
Additional information
Part V of: Halogenometalates of Transition Elements with Cations of N-heterocyclic Bases, Part IV:[1]
The financial support by the Deutsche Forschungsgemeinschaft is gratefully acknowledged. The technical assistance of Margit Baumbach is gratefully acknowledged as well.
Rights and permissions
About this article
Cite this article
Feist, M., Kunze, R., Neubert, D. et al. The thermal behavior of triethylenediammonium salts. Journal of Thermal Analysis 49, 635–647 (1997). https://doi.org/10.1007/BF01996746
Issue Date:
DOI: https://doi.org/10.1007/BF01996746