Abstract
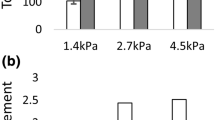
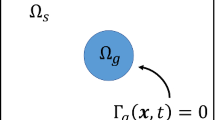
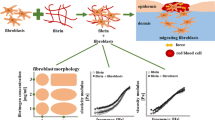
Traction forces developed by most cell types play a significant role in the spatial organisation of biological tissues. However, due to the complexity of cell-extracellular matrix interactions, these forces are quantitatively difficult to estimate without explicitly considering cell properties and extracellular mechanical matrix responses. Recent experimental devices elaborated for measuring cell traction on extracellular matrix use cell deposits on a piece of gel placed between one fixed and one moving holder. We formulate here a mathematical model describing the dynamic behaviour of the cell-gel medium in such devices. This model is based on a mechanical force balance quantification of the gel visco-elastic response to the traction forces exerted by the diffusing cells. Thus, we theoretically analyzed and simulated the displacement of the free moving boundary of the system under various conditions for cells and gel concentrations. This modelis then used as the theoretical basis of an experimental device where endothelial cells are seeded on a rectangular biogel of fibrin cast between two floating holders, one fixed and the other linked to a force sensor. From a comparison of displacement of the gel moving boundary simulated by the model and the experimental data recorded from the moving holder displacement, the magnitude of the traction forces exerted by the endothelial cell on the fibrin gel was estimated for different experimental situations. Different analytical expressions for the cell traction term are proposed and the corresponding force quantifications are compared to the traction force measurements reported for various kind of cells with the use of similar or different experimental devices.
Similar content being viewed by others
REFERENCES
Barocas, V.H., A.G. Moon and R.T. Tranquillo (1995). The fibroblast-populated collagen microsphere assay of cell traction force: part 2. Measurement of the cell traction parameter. J. Biomech. Engin. 117: 161–170.
Bell, E., B. Ivarsson and C. Merrill (1979). Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 76: 1274–1278.
Benkherourou, M., P.Y. Gumery, C. Rochas and M. Asfour (1997). Characterisation method for low concentration biogels. (in preparation).
Chapuis, J.F. and P. Agache (1992). A new technique to study the mechanical properties of collagen tattices. J. Biomech. 25: 115–120.
Clark, R.A. and P.M. Henson (1988). The Molecular and Cellular Biology of Wound Repair. New York, Plenum.
Cook, J. (1995). Mathematical models for dermal wound bealing: wound contraction and scar formation. PhD thesis, University of Washington.
Cruywagen, G.C. and J.D. Murray (1992). On a tissue interaction model for skin pattern formation. J. Nonlinear Sci. 2: 217–240.
Delvoye, P., P. Wiliquet, J.L. Levêque, B.V. Nusgens, and C.M. Lapière (1991). Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J. Invest. Dermatol. 97: 898–902.
Djabourov, M. and J.M. Cuenet (1995). Les gels, des liquides qui ne coulent pas. Pour la science 215: 50–57.
Eastwood, M., R. Porter, U. Khan, G. McGrouther and R. Brown (1996). Quantitative analysis of coilagen gel contractile forces generated by dermal fibroblasts and the relationship to cell morphology. J. Cell Phys. 166: 33–42.
Eastwood, M., D.A. McGrouther and R.A. Brown (1994). A culture force monitor for measurement of contraction forces generated in human dermal fibroblast cultures: evidence for cell-matrix mechanical signalling. Bioch. Biophys. Acta. 1201: 186–192.
Ehrlich, H.P. (1988) The role of connective tissue matrix in would healing. Prog. Clin. Biol. Res. 266: 243–258.
Ehrlich, H.P. and J.B. Rajaratham (1990). Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell 22: 407–417.
Es Salhiene, M., B. Vailhé, P. Tracqui, P.Y. Gumery and L. Tranqui (1995). Measurement of the forces exerted by endothelial cells seeded onto fibrin gels. (Abstract) J. Mol. Cell Cardiol. 7: 27 A 462.
Ferry, J.D. (1988). Structure and rheology of fibrin networks. In: O. Kramer, ed., Biological and Synthetic Polymer Networks. London, Elsevier.
Fung, Y.C. (1993). Biomechanics. Mechanical Properties of Living Tissue. Berlin, Springer Verldg.
Guenet, J.M. (1992) Thermoreversible gelation of polymers and biopolymers. London, Acad, Press.
Guidry, C. and F. Grinnell (1986). Contraction of hydrated collagen gels by fibroblasts: evidence for two mechanisms by which collagen fibrils are stabilized. Collagen. Rel. Res. 6: 515–529.
Harris, A.K., D. Stopak and P. Wild (1981). Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 290: 249–251.
Harris, A.K. (1984). Tissue culture cells on deformable substrata: biomechanical implications. J. Biomech. Engin. 106: 19–24.
Hynes, R.O. (1992). Integrins: versatility, modulation, and signalling in cell adhesion. Cell 69: 11–25.
Ingber, D. (1991) Integrins as mechanochemical transducers. Current Opinion in Cell Biol. 3: 841–848.
Ingber, D.E. (1993) Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J. Cell Sci. 104: 613–627.
Jain, M.K., R.A. Berg, and G.P. Tandon (1990) Mechanical stress and cellular metabolism in living soft tissue composites. Biomaterials 11: 465–472.
Juliano, R.L. and S. Haskill (1993). Signal transduction from the extracellular matrix. J. Cell Biol. 120: 577–585.
Kolodney, M.S. and R.B. Wysolmerski (1992). Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J. Cell Biol. 117: 73–82.
Landau, L. and E. Lifshitz. (1990). Théorie de I'Élasticité. 2nd edition.
McGrath, M.H. and R.H. Simon (1983). Wound geometry and the kinetics of wound contraction. Plast. Reconstr. Surg. 72: 66–73.
Moon, A.G. and R.T. Tranquillo (1993). Fibroblast-populated collagen microsphere assay of cell traction torce: Part 1. Continuum model. AlChE J. 39: 163–177.
Murray, J.D. and G.F. Oster (1984). Cell traction models for generating pattern and form in morphogenesis. J. Math. Biology. 19: 265–279.
Murray, J.D. (1993). Mathematical biology. 2nd edition. Springer-Verlag.
Olsen, L., J.A. Sherratt and P.K. Maini (1995). A mechanochemical model for adult dermal wound contraction and the permanence of the contracted tissue displacement profile. J. Theor. Biol. 177: 113–128.
Oster, J.D. Murray and A.K. Harris (1983). Mechanical aspects of mesenchymal morphogenesis. J. Embryol. Exp. Morph. 78: 83–125.
Özerdem, B. and A. Tözeren (1995). Physical response of collagen gels to tensile strain. J. Biomech. Eng. 117: 397–401.
Scherer, G.W., H. Hdach and J. Phalippou (1991). Thermal expansion of gels: a novel method for measuring permeability. J. Non. Cryst. Solids. 130: 157–170.
Stopak, D. and A.K. Harris (1982). Connective tissue morphogenesis by fibroblast traction. Dev. Biol. 90: 383–398.
Tracqui, P., D.E. Woodward, G.C. Cruywagen, J. Cook and J.D. Murray (1995). A mechanical model for fibroblast-driven wound healing. J. Biol. Syst. 3: 1075–1084.
Tranquillo, R.T. and J.D. Murray (1992). Continuum model of fibroblast-driven wound contraction: inflammation-mediation. J. Theor. Biol. 158: 135–172.
Trinkaus, J.P. (1984) From Cells into Organs. 2nd edition. Englewood Cliffs, Prentice-Hall.
Vailhé, B., X. Ronot, P. Tracqui, Y. Usson and L. Tranqui (1997). In vitro angiogenesis is modulated by the mechanical properties of fibrin gels and is related to α2β3 integrin localisation. In Vitro Cell. Dev. Biol. (in press).
Yamato, M., E. Adachi, K. Yamamoto and T. Hayashi (1995). Condensation of collagen fibrils to the direct vicinity of fibroblasts as a cause of gel contraction. J. Biochem. 117: 940–946.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ferrenq, I., Tranqui, L., Vailhé, B. et al. Modelling Biological Gel Contraction by Cells: Mechanocellular Formulation and Cell Traction Force Quantification. Acta Biotheor 45, 267–293 (1997). https://doi.org/10.1023/A:1000684025534
Issue Date:
DOI: https://doi.org/10.1023/A:1000684025534