Summary
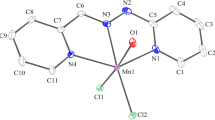
The complexes [MoL*(NO)Cl(YC6H4YH-m)] (Y = O or NH), [MoL*(NO)Cl(YC10H6YH-1,5)], (Y = O or NH), [MoL*(NO)Cl(OC10H6OH-2,7)], [{MoL*(NO)Cl}2(XC6H4Y-m)] (X=Y=O, NH or S; X=O, Y=NH), [{MoL*(NO)-C1}2(YC10H6Y-1,5)] (Y=O or NH) and [{MoL*(NO)Cl2-(OC10H6-2,7)] have been prepared and studied by cyclic voltammetry. The monometallic species undergo a reversible oneelectron reduction, whereas the bimetallics undergo two oneelectron reductions. A comparison of ΔE1/2 (E1/2(1)-E1/2(2)) values for those new species with those obtained frompara- substituted analogues and bimetallics containing extended bridges YC6H4ZC6H4Y (e.g. Z = S or CH2CH2) established that the interaction between the redox centres in these new species is intermediate (YC6H4Y-m; NHC10H6NH-1,5) or weak (OC10H6O).
In earlier papers1,2 we have described the synthesis and electrochemical properties of a series of mono- and bi-metallic complexes of the type [MoL*(NO)X(YC6H4YH)], [MoL*(NO)X}2(YC6H4Y)] and [{MoL*(NO)X}2(YC6H4-ZC6H4Y)] [L*=tris(3,5-dimethylpyrazolyl)borate, HB(Me2C3HN2)3] where the arene ring ispara-substituted (X=Cl or I while Y=O, S or NH and Z = nothing, CH2, CH2CH2, S, SO2 or O). We have shown that the E1/2-values of these species are dependent on X and Y, and that the bimetallic species undergo two one-electron reduction processes.
We have established that there is strong interaction between the redox centres in bimetallics bridged byp-YC6H4Y, but that weak-to-negligible interaction occurs in those species containing YC6H4ZC6H4Y bridges. In this paper we describe our investigations ofmete-substituted bridging systems,m-YC6H4Y, and comparable systems containing naphthalene bridges,e.g. 1,5- or 2,7-YC10H2Y. From these studies we hoped to establish the extent of interaction between the two redox centres and how this compared to thepara-substituted arene counterparts.
Similar content being viewed by others
References
S. M. Charsley, C. J. Jones, J. A. McCleverty, B. D. Neaves and S. J. Reynolds,J. Chem. Soc., Dalton Trans., (1986), submitted.
S. M. Charsley, C. J. Jones, J. A. McCleverty, B. D. Neaves and S. J. Reynolds,J. Chem. Soc., Dalton Trans., (1986), submitted.
A. S. Drane and J. A. McCleverty,Polyhedron, 2, 53 (1983).
J. A. McCleverty, A. S. Drane, N. A. Bailey and J. M. A. Smith,J. Chem. Soc., Dalton Trans., 91 (1983).
N. Al-Obaidi, S. M. Charsley, C. J. Jones, J. A. McCleverty, B. D. Neaves and S. J. Reynolds, in preparation.
J. A. McCleverty, D. Seddon, N. A. Bailey and N. W. Walker,J. Chem. Soc., Dalton Trans., 898 (1976); S. Trofimenko,J. Am. Chem. Soc., 89, 3904 (1967) andIbid, 91, 588 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Charsley, S.M., Jones, C.J. & McCleverty, J.A. Mono- and bi-metallic complexes based on redox-active tris(3,5-dimethylpyrazolyl)borato-molybdenum nitrosyls, part III.(1) Complexes containing meta-substituted benzene- and naphthalenediols or -diamines. Transition Met Chem 11, 329–334 (1986). https://doi.org/10.1007/BF00621516
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00621516