Summary
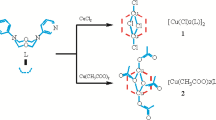
The monomer in the title was obtained as a first crop and the dimer as a second crop from a 95% ethanolic solution of CuCl2 and 5,5′-6,6′-tetrahydro-2,2′-bi-4H-1,3-thiazine (2,2′-bi-2-thiazine) in the mole ratio 1∶1.1. The monomeric crystals are blue, dichloro(5,5′-6,6′-tetrahydro-2,2′-bi-4H-1, 3-thiazine- N 3,N 3′)copper(II).
The crystal consists of monomeric molecules with no short contacts to neighbouring molecules. The Cu atoms occupy special positions on 2-fold axes. The bidentate 2,2′-bi-2-thiazine bonds via N as it assumes a cis configuration with a twist of 10.3° about the inter-ring C—C bond. The Cu—N distance is 2.013(5) Å, and Cu—Cl is 2.231(2) Å. Coordination around Cu is slightly distorted square planar with a dihedral angle of 5.8(2)° between the Cu, N, Cl plane and Cu, N′, Cl′ plane. The dimeric crystals are green, di-μ-chloro-bis[chloro(5,5′-6,6′-tetrahydro-2,2′-bi-4H-1, 3-thiazine-N 3,N 3′)copper(II)].
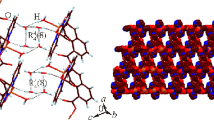
The structure consists of discrete centrosymmetric dimers [CuCl2(C8H12N2S2)]2. Coordination around Cu is distorted square pyramidal (τ = 0.32) with two N atoms from the bidentate 2,2′-bi-2-thiazine occupying adjacent basal sites, and two Cl atoms occupying the other two basal sites. The average Cu—N distance is 2.028(4) Å, Cu—Cl(1) is 2.284(2) Å, Cu—Cl(2) is 2.239(1) Å. Dihedral angle between the CuN2 and CuCl2 planes is 23.7°. Cl(1)′ of the adjacent inversion-related monomer, CuCl2-(C8H12N2S2), occupies the apical position. Cu—Cl(1)′, r = 2.618(2) Å. Dimensions in the chloride-bridged planar dimeric moiety, Cu2Cl2, are Cu ⋯ Cu′ 3.449(1) Å; Cl(1) ⋯ Cl(1)′ 3.498(2) Å; Cu—Cl(1)—Cu′, φ = 89.2(1)°, φ/r = 34.07°/Å. The two saturated rings in C8H12N2S2 show a twist of 11.4° about the inter-ring C—C bond.
Similar content being viewed by others
References
D. A. Tomalia and J. N. Paige, J. Org. Chem., 38, 3949 (1973).
R. S. Glass, M. Sabahi, M. Hojjatle and G. S. Wilson, Inorg. Chem., 26, 2194 (1987).
J. G. Haasnoot, W. L. Driessen and J. Reedijk, Inorg. Chem., 23, 2803 (1984).
J. Nelson, S. M. Nelson and W. D. Perry, J. Chem. Soc., Dalton Trans., 1282 (1976).
R. A. Johnson, Diss. Abstr. Int. B, 43, 1841 (1982).
J. C. Huffman and A. P. Sattelberger, Cryst. Struct. Commun., 10, 1535 (1981).
M. G. B. Drew, T. R. Pearson, B. P. Murphy and S. M. Nelson, Polyhedron, 2, 269 (1983).
R. A. Johnson, R. B. von Dreele and T. M. Brown, Inorg. Chem., 23, 4302 (1984).
W. E. Hatfield, in L. V. Interrate (Ed.), Extended Interaction Between Metal Ions, ACS Symp. Ser., 5, 108 (1974).
D. J. Hodgson, Prog. Inorg. Chem., 19, 173 (1975).
P. J. Hay, J. C. Thibeault and R. Hoffmann, J. Am. Chem. Soc., 97, 4884 (1975).
V. F. Duckworth, D. P. Graddon, N. C. Stephenson and E. C. Watton, Inorg. Nucl. Chem. Lett., 3, 557 (1967).
D. H. Svedung, Acta Chem. Scand., 23, 2865 (1969).
N. T. Watkins, E. E. Dixon, V. H. Crawford, K. T. McGregor and W. E. Hatfield, J. Chem. Soc., Chem. Commun., 133 (1973).
V. F. Duckworth and N. C. Stephenson, Acta Crystallogr., B25, 1995 (1969).
D. Y. Jetter, D. J. Hodgson and W. E. Hatfield, Inorg. Chim. Acta, 5, 257 (1971).
W. E. Marsh, W. E. Hatfield and D. J. Hodgson, Inorg. Chem., 21, 2679 (1982).
E. Stetten and A. Apeland, Acta Crystallogr., B31, 2019 (1975).
E. D. Estes, W. E. Estes, W. H. Hatfield and D. J. Hodgson, Inorg. Chem., 14, 106 (1975).
D. W. Phelps, W. H. Goodman and D. J. Hodgson, Inorg. Chem., 15, 2266 (1976).
P. G. Beckingsale, A. T. Morcom, C. E. F. Rickard and T. N. Waters, J. Chem. Soc., Dalton Trans., 2135 (1977).
G. R. Desiraju, H. R. Luss and D. L. Smith, J. Am. Chem. Soc., 100, 6375 (1978).
B. Cohen, C. C. Ou, R. A. Lalancette, W. Borowski, J. A. Potenza and H. J. Schugar, Inorg. Chem., 18, 217 (1979).
D. D. Swank, G. F. Needham and R. D. Willett, Inorg. Chem., 18, 761 (1979).
M. R. Churchill and J. P. Hutchinson, Cryst. Struct. Commun., 9, 1209 (1980).
S. P. Knap, B. H. Toby, M. Sebastian, K. Krogh-Jespersen and J. A. Potenza, J. Org. Chem., 46, 2490 (1981).
M. Mégnamisi-Bélombé and H. Endres, Acta Crystallogr., C39, 707 (1983).
F. Nepveu, F. J. Bormuth and L. Walz, J. Chem. Soc., Dalton Trans., 1213 (1986).
G. DeMunno, G. Denti and P. Dapporto, Inorg. Chim. Acta, 74, 199 (1983).
J. A. Carrabine and M. Sundaralingam, J. Am. Chem. Soc., 92, 369 (1970).
M. Sundaralingam and J. A. Carrabine, J. Mol. Biol., 287 (1971).
J. P. Declercq, M. Debbaudt and M. Van Meerssche, Bull. Soc. Chim. Belg., 80, 527 (1971).
R. F. Drake, V. H. Crawford, N. W. Laney and W. E. Hatfield, Inorg. Chem., 13, 1246 (1974).
D. J. Hodgson, P. K. Hale and W. E. Hatfield, Inorg. Chem., 10, 1061 (1971).
K. T. McGregor, D.B. Losee, D. J. Hodgson and W. E. Hatfield, Inorg. Chem., 13, 756 (1974).
W. E. Marsh, K. C. Patel, W. E. Hatfield and D. J. Hodgson, Inorg. Chem., 22, 511 (1983).
R. E. Norman, N. J. Rose and R. E. Stenkamp, Acta Crystallogr., C46, 1 (1990).
J. D. Dunitz, Acta Crystallogr., 10, 307 (1957).
M. Mágnamisi-Bélombé, P. Singh, D. E. Bolster and W. E. Hatfield, Inorg. Chem., 23, 2578 (1984).
H. Endres, Acta Crystallogr., C39, 1192 (1983).
J. A. Bertrand and J. A. Kelly, J. Am. Chem. Soc., 88, 4746 (1966).
T. Kilborn and J. D. Dunitz, Inorg. Chim. Acta, 1, 209 (1967).
J. Pickardt and N. Rautenberg, Z. Naturforsch., 37b, 1355 (1982).
J. T. Guy, Jr., J. C. Cooper, R. D. Gilardi, J. L. Flippen-Anderson and C. F. George, Jr., Inorg. Chem., 27, 635 (1988).
A. Erdonmez, J. H. Van Diemen-Rudolf, A. G. De Graaff and J. Reedijk, Acta Crystallogr., C44, 402 (1990).
N. Walker and D. Stuart, Acta Crystallogr., A39, 158 (1983).
G. M. Sheldrick, SHELX-86, Program for Crystal Structure Determination University of Göttingen, 1986.
G. M. Sheldrick, SHELX-76, Program for Crystal Structure Determination, University of Cambridge, 1976.
A. L. Spek in D. Sayre (Ed.), Computational Crystallography, Clarendon Press, Oxford, 1982, p. 528.
C. K. Johnson, ORTEPII, Report ORNL-5138, Oak Ridge National Laboratory, Tennessee, 1976.
W. D. S. Motherwell and W. Clegg, PLUTO, University ofCambridge, 1978.
P. J. Burke, K. Henrick and D. R. McMillin, Inorg. Chem., 21, 1881 (1982).
A. Lavery, S. M. Nelson and M. G. B. Drew, J. Chem. Soc., Dalton Trans., 2975 (1987).
J. Lisowski, M. Grzeszezuk and L. Latos-Grażynski, Inorg. Chim. Acta, 161, 153(1989).
P. M. Colman, H. C. Freeman, J. M. Guss, M. Murata, V. A. Nords, J. A. M. Ramshaw and M. P. Venkatappa, Nature, 272, 319 (1978).
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1349 (1984).
R. D. Shannon, Acta Crystallogr., A32, 751 (1976).
D. J. Hodgson and E. Pederson, Acta Chem. Scand., A36, 281 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Haddad, S.F., Pickardt, J. Polymerized isomers of CuCl2(C8H12N2S2). The X-ray crystal structures of dichloro(5,5′-6,6′-tetrahydro-2,2′-bi-4H-1,3-thiazine-N 3, N 3′)copper(II) and di-μ-chloro-bis[chloro(5,5′-6,6′-tetrahydro-2, 2′-bi-4H-1,3-thiazine-N 3,N 3′)copper(II)]. Transition Met Chem 18, 377–384 (1993). https://doi.org/10.1007/BF00208176
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00208176