Summary
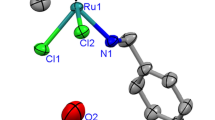
The degradation-resistant ligand tri-2-pyridylamine (tripyam) (1) forms a variety of stable ruthenium complexes. Reaction of (1) with RuCl2(PPh3)3 yields the complex RuCl2(PPh3)(tripyam) (2) and, upon prolonged heating in pyridine, forms RuCl2(py)(tripyam) (3). Complexes (2) and (3) display unusual thermal stability, resisting degradation at temperatures of 270 °C. Reaction of (2) with two equivalents of AgSbF6 in water yields the solvento complex [Ru(PPh3)(tripyam)(OH2)2] (SbF6)2 (2a). Reaction of (1) with RuCl3·H2O also yields the trichloro complex RuCl3(tripyam) (4). The organometallic precursor [RuCl2(p-cymene)]2 reacts with (1) and either two or four equivalents of AgSbF6 to yield [RuCl(p-cymene)(η 2-tripyam)]SbF6 (5) and [Ru(p-cymene) (η 3-tripyam)](SbF6)2 (6), respectively. Each of these complexes has been characterized by spectroscopic techniques and, in the case of (5), by single-crystal X-ray diffraction.
Similar content being viewed by others
References
G. Wilkinson, F. G. A. Stone and E. W. Abel (Eds), Comprehensive Organometallic Chemistry, Pergamon Press, Oxford, 1982, Vol. 4, section 32.9.
L. Roecker, J. C. Dobson, W. J. Vining and T. J. Meyer, Inorg. Chem., 26, 779 (1987); L. Roecker and T. J. Meyer, J. Am. Chem. Soc., 109, 746 (1987); T. C. Dobson, W. K. Seok and T. J. Meyer, Inorg. Chem., 25, 1514 (1986); K. J. Takeuchi, G. J. Samuel, S. W. Gersten, J. A. Gilbert and T. J. Meyer, Inorg. Chem., 22, 1407 (1983); B. A. Mayer and T. J. Meyer, Inorg. Chem., 20, 436 (1981); J. C. Dobson and T. J. Meyer, Inorg. Chem., 27, 3283 (1988); J. C. Dobson and T. J. Meyer, Inorg. Chem., 28, 2013 (1989); O. Geselowitz and T. J. Meyer, Inorg. Chem., 29, 3894 (1990); A. Dovletoglou, S. A. Adeyemi, M. H. Lym, D. J. Hodgson and T. J. Meyer, J. Am. Chem. Soc., 112, 8989 (1990); C. D. Ellis, J. A. Gilbert, W. R. Murphy and T. J. Meyer, J. Am. Chem. Soc., 105, 4842 (1983); A. Llobet, D. J. Hodgson and T. J. Meyer, Inorg. Chem., 29, 3760 (1990); J. C. Dobson, J. H. Helms, P. Doppelt, B. P. Sullivan, W. E. Hatfield and T. J. Meyer, Inorg. Chem., 28, 2200 (1989); J. Gilbert, L. Roecker and T. J. Meyer, Inorg. Chem., 26, 1126 (1987); J. C. Dobson, K. J. Takeuchi, D. W. Pipes, D. A. Geselowitz and T. J. Meyer, Inorg. Chem., 25, 2357 (1986); R. A. Leising and K. J. Takeuchi, Inorg. Chem., 26, 4391 (1987); T. C. Lau, C. M. Che, W. O. Lee and C. K. Poon, J. Chem. Soc., Chem. Commun., 1406 (1988); C. M. Che, W. H. Leung, C. K. Li and C. K. Poon, J. Chem. Soc., Dalton Trans., 379 (1991); C. M. Che and W. O. Lee, J. Chem. Soc., Chem. Commun., 881 (1988); C. M. Che, K. Lau, T. C. Lau and C. W. Poon, J. Am. Chem. Soc., 112, 5176 (1990); J. G. Muller, J. H. Acquaye and K. J. Takeuchi, Inorg. Chem., 31, 4552 (1992).
K. K. Mosny and R. H. Crabtree, Inorg. Chim. Acta, in the press.
P. S. Hallman, T. A. Stephenson, Inorg. Synth., 12, 237 (1970).
M. A. Bennett, A. K. Smith, J. Chem. Soc., Dalton Trans., 233 (1974).
B. P. Sullivan, J. M. Calvert and T. J. Meyer, Inorg. Chem., 19, 1404 (1980).
R. Tanke, S. E. M. Holt and R. H. Crabtree, Inorg. Chem., 30, 1714 (1991).
R. J. Restivo, G. Ferguson, D. J. O'Sullivan and F. J. Lalor, Inorg. Chem., 14, 3046 (1975).
E. S. Kucharski, W. R. McWhinnie and A. H. White, Aust. J. Chem., 31, 53 (1978); E. S. Kucharski, W. R. McWhinnie and A. H. White, Aust. J. Chem., 31, 2647 (1978); P. L. Dedert, T. Sorrell, T. J. Marks and J. A. Ibers, Inorg. Chem., 21, 3056 (1982); P. A. Anderson, F. R. Keene, E. Horn and E. R. T. Tiekink, Appl. Organometal. Chem., 4, 523 (1990); M. R. Churchill, G. Davies, M. A. El-Sayed, M. F. El-Shalzy, J. P. Hutchinson and M. W. Rupich, Inorg. Chem., 19, 201 (1980); F. R. Keene, M. P. Snow, P. J. Stephenson and E. R. T. Tiekink, Inorg. Chem., 27, 2040 (1988); P. L. Dedert, J. S. Thompson, J. A. Ibers and T. J. Marks, Inorg. Chem., 21, 969 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mosny, K.K., de Gala, S.R. & Crabtree, R.H. Arene ruthenium complexes of the thermally resistant ligand tri-2-pyridylamine. Transition Met Chem 20, 595–599 (1995). https://doi.org/10.1007/BF00136427
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00136427