Abstract
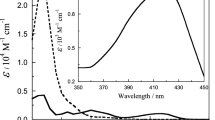
The anation kinetics of the protonated dioxotetracyanomolybdate(IV) ion with 1,10-phenanthroline have been studied spectrophotometrically. The effect of the H+ ion, ionic strength and temperature on the reaction rate has been determined; the rate increases with increasing H+ ion concentration, is independent of ionic strength, and increases with temperature. The reaction follows first order kinetics with respect to [Mo(OH)2- (H2O)2(CN)4]2− and is considered to proceed through the formation of a 1,10-phenanthroline–Mo(OH)2- (H2O)2(CN)4M2− complex (outer sphere) which converts into an inner sphere complex. The formation constant (Kn) for the outer sphere complex has been calculated from the kinetic data by two different methods. Anation of Mo(OH)2-(H2O)2(CN)42− is discussed in terms of an associative interchange (Ia) mechanism. The activation parameters have been calculated using the Arrhenius equation. A substitution mechanism is proposed and the rate equation derived:
kobs = kKnKa[H+] [phen] /{1 + KnKa[H+] [phen]}.
Similar content being viewed by others
References
S. J. Lippard and B. J. Russ, Inorg. Chem., 6, 1943 (1967).
S. J. Lippard, B. J. Russ and N. Nozaki, Chem. Commun., 3, 119 (1967).
J. Van de Poell and H. M. Neumann, Inorg. Chem., 7, 2086 (1968).
R. K. Murman and R. P. Robinson, J. Inorg. Nucl. Chem., 14, 203 (1975).
A. Kanas, M. Dudek and A. Samotus, Bull. Acad. Pol. Sci. Ser. Sci. Chem., 24, 43 (1976).
M. Dudek, A. Kanas and A. Samotus, J. Inorg. Nucl. Chem., 42, 187 (1980).
S. S. Basson, J. G. Leipoldt and I. M. Potgieter, Inorg. Chim. Acta, 87, 71 (1984).
S. S. Basson, J. G. Leipoldt and I. M. Potgieter, Inorg. Chim. Acta, 90, 57 (1984).
S. S. Basson, J. G. Leipoldt and I. M. Potgieter, Inorg. Chim. Acta, 103, 121 (1985).
D. Benerjee and J. Roy, Z. Anorg. Allg. Chem., 399, 115 (1969).
J. N. Mandal and G. S. De, Indian J. Chem, 16A, 580 (1978).
B. A. N. Murty, K. V. Subbaiah and P. V. Subba Rao, Indian J. Chem., 18A, 261 (1979).
K. S. Upadhya and M. C. Agarwal, Indian J. Chem., 19A, 478 (1980).
C. Chatterjee and A. K. Basak, Indian J. Chem., 16A, 758 (1978).
C. H. Longford and H. B. Gray, Ligand Substitution Processes, Wiley, New York, 1965, p.8.
S. I. Ali and H. Kaur, P. Photochem. Photobiol., A 61, 183 (1991).
S. I. Ali and H. Kaur, P. Photochem. Photobiol, 68, 147 (1992).
S. I. Ali and H. Kaur, Transition Met. Chem., 16, 450 (1991).
M. A. Beg, Kabiruddin and R. A. Khan, Aust. J. Chem., 26, 671 (1973).
A. W. Adamson and J. R. Perumareddi, Inorg. Chem., 4, 247 (1965).
M. Eigen and K. Tamm, Z. Elektron Chem, 66, 93, 107 (1962).
R. A. Plane and H. Taube, J. Phys. Chem., 56, 33 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ali, S.I., Khan, Z. & Murtaza, Z. Kinetics and mechanism of the complexation and anation of protonated dioxotetracyanomolybdate(IV) with 1,10-phenanthroline. Transition Metal Chemistry 22, 79–83 (1997). https://doi.org/10.1023/A:1018434206779
Issue Date:
DOI: https://doi.org/10.1023/A:1018434206779