Abstract
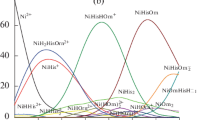
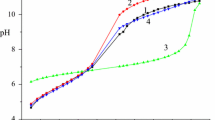
The kinetics of the complexation of NiII by pteroylglutamic acid have been studied in the 545 ∘C range, the ionic strength (0.6 M) being regulated with KNO3, in the 5.5–7.0pH range, using the stopped-flow method. Under the experimental conditions two processes were observed. The faster process was detected in the millisecond range and is associated with the reaction between NiII and the ligand. The slower is observed within a few seconds. Complementary equilibrium studies were made at 25 ∘C. The results are consistent with the formation of a 1:1 complex between the reactants, and a mechanism is proposed to account for the observed behaviour. Equilibrium constants for the NiII plus pteroylglutamic acid system, as well as activation parameters, are reported.
Similar content being viewed by others
References
R. Nayan and A. K. Dey, Z. Naturforsch., Teil B, 52, 1453 (1970).
M. Poe, J. Biol. Chem., 252, 3724 (1977).
M. Poe, J. Biol. Chem., 248, 7025 (1973).
A. Albert, Biochem J., 54, 646 (1953).
J. T. H. Roos and D. R. Williams, J. Inorg. Nucl. Chem., 39, 367 (1977).
A. H. Thomas, M. R. Feliz and A. L. Capparelli, Transition Met. Chem., 21, 317 (1996).
P. K. Chattopadhyay and B. Kratochvil, Can. J. Chem., 54, 2540 (1976).
P. K. Chattopadhyay and B. Kratochvil, Inorg. Chem., 18, 2953 (1979).
R. G. Wilkins, Comments Inorg. Chem., 2, 187 (1983).
R. G. Wilkins, Acc. Chem. Res., 3, 408 (1970).
D. M. W. Buck and P. Moore, J. Chem. Soc., Dalton Trans., 2082 (1974).
R. B. Jordan and B. E. Erno, Inorg. Chem., 18, 2895 (1979).
R. L. Reeves, G. S. Calabrese and S. A. Harkaway, Inorg. Chem., 22, 3076 (1983).
R. G. Wilkins, Kinetics and Mechanism of Reactions of Transition Metal Complexes, 2nd Edit, VCH, New York, 1991 p. 219.
R. M. Fuoss, J. Am. Chem. Soc., 80, 5059 (1958).
A. E. Mantell and R. M. Smith, Critical Stability Constants, Plenum Press, New York, 1975, Vol. 2, p. 165.
Y. Ducommun, K. E. Newman and A. E. Merbach, Inorg. Chem., 19, 3696 (1980).
Y. Ducommun, D. Zbinden and A. E. Merbach, Helv. Chim. Acta, 65, 1385 (1982).
P. Bernhard, L. Helm, I. Rapaport, A. Ludi and A. E. Merbach, Inorg. Chem., 27, 873 (1988).
E. A. Miromov and V. S. Nabokov, Khim.-Farm. Zh., 10, 136 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thomas, A., Wolcan, E., Fe´liz, M.R. et al. Kinetic study of nickel(II) complexation by pteroylglutamic acid. Transition Metal Chemistry 22, 541–544 (1997). https://doi.org/10.1023/A:1018544220350
Issue Date:
DOI: https://doi.org/10.1023/A:1018544220350