Abstract
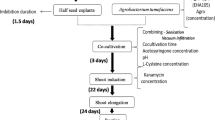
An efficient protocol for Agrobacterium tumefaciens-mediated transformation of six commercial Brassica napus winter cultivars is described. Two B. napus spring cultivars were analysed for comparison. Five strains of A. tumefaciens with different combinations of nopaline and octopine chromosomal backgrounds and virulence plasmids were used for cocultivation. Selection of putative regenerated transgenic plants was performed on kanamycin- or hygromycin-containing media. The scores of transgenic plants were calculated on the basis of GUS (β-glucuronidase) activity, detected by the histochemical X-Gluc test. Target tissue derived from the cut surface of cotyledon petioles resulted in successful transformation with all the winter cultivars tested. Target tissue from hypocotyl segments resulted in a successful transformation with only one winter cultivar. The transformation rates for B. napus winter cultivars in this study were higher than in previous reports. Southern blot analysis revealed that integration of marker genes occurred in single and in multiple copies and at multiple loci in the genome. The transgenic plants all grew normally and developed fertile flowers after a vernalization period. After self-pollination, Southern blot analysis of selected GUS active F1 plants revealed that introduced marker genes were stably inherited to the next generation. These data demonstrate that morphologically normal, fertile transgenic plants of B. napus winter cultivars can be achieved with both nopaline- and octopine-derived A. tumefaciens strains. This protocol should have a broad application in improvement of Brassica napus winter cultivars by introduction of foreign genes
Similar content being viewed by others
References
Altenbach, S.B., Kuo, C.C., Staraci, L.C., Pearson K.W., Wainwright, C., Georgescu, A. and Townsend, J. (1992) Accumulation of a Brazil nut albumin in seeds of transgenic canola results in enhanced levels of speed protein methionine. Plant Mol. Biol. 18, 235-46.
Boulter, M.E., Croy, E., Simpson, P., Shields, R., Croy, R.R. D. and Shirsat, A.H. (1990) Transformation of Brassica napus(oilseed rape) using Agrobacterium tumefaciensand Agrobacterium rhizogenes- a comparison. Plant Sci. 70, 91-9.
Charest, P.J., Holbrook, L.A., Gabard, J., Iyer, V.N. and Miki, B.L. (1988) Agrobacterium-mediated transformation of thin cell layer explants from Brassica napusL. Theor. Appl. Genet. 75, 438-45.
Chen, J.L. and Beversdorf, W.D. (1994) A combined use of microprojectile bombardment and DNA imbibition enhances transformation frequency of canola (Brassica napusL.). Theor. Appl. Genet. 88, 187-92.
Damgaard, O., and Rasmussen, O. (1991) Direct regeneration of transformed shoots in Brassica napusfrom hypocotyl infections with Agrobacterium rhizogenes. Plant Mol. Biol. 17, 1-8.
De Block, M., Debrouwer, D. and Tenning, P. (1989) Transformation of Brassica napusand Brassica oleraceausing Agrobacterium tumefaciensand the expression of the barand neogenes in the transgenic plants. Plant Physiol. 91, 694-701.
De Block, M. and Debrouwer, D. (1991) Two T-DNA’s cotransformed into Brassica napusby double Agrobacterium tumefaciensinfection are mainly integrated at the same locus. Theor. Appl. Genet. 82, 257-63.
Dellaporta, S.L., Wood, J., and Hicks, J.B. (1984) A plant DNA minipreparation. In: A Laboratory Course Manual, Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, pp. 36-7.
Depicker, A., Stachel, S., Dhaese, P., Zambryski, P. and Goodman, H.M. (1982) Nopaline synthase: Transcript mapping and DNA sequence. J. Mol. Appl. Genet. 1, 561-73.
Downey, R.K. and Röbbelen, G. (1989) Brassicaspecies. In: Röbbelen, G., Downey, R. K. and Ashri, A. eds, Oil Crops of the World, New York, NY: McGraw-Hill Inc., pp. 339-62.
Fry, J., Barnason, A. and Horsch, R.B. (1987) Transformations of Brassica napuswith Agrobacterium tumefaciensbased vectors. Plant Cell Rep. 6, 321-5.
Herrera-Estrella, L., De Block, M., Messens, E., Hernalsteens, J.P., Van Montagu, M. and Schell, J. (1983) Chimeric genes as dominant selectable markers in plant cells. EMBO J. 2, 987-95.
Hollbrook, L.A. and Miki, B.L. (1985) Brassicacrown gall tumourigenesis and in vitroculture of transformed tissue. Plant Cell Rep. 4, 329-32.
Jefferson, R.A., Kavanagh, T.A. and Bevan, M.W. (1987) GUS fusions: β-glucuronidase as a sensitive and versaltile gene fusion marker in higher plants. EMBO J. 6, 3901-7.
Khehra, G.S. and Mathias, R.J. (1992) The interaction of genotype, explant and media on the regeneration of shoots from complex explants of Brassica napusL. J. Exp. Bot. 43, 1413-8.
Knutzon, D.S., Thompson, G.A., Radke, S.E., Johnson, W.B., Knauf, V.C. and Kridl, J.C. (1992) Modification of Brassicaseed oil by antisense expression of a stearoyl-acyl carrier protein desaturase gene. Proc. Natl Acad. Sci. USA 89, 2624-8.
Kosugi, S., Ohashi, Y., Nakajima, K. and Arai, Y. (1990) An improved assay for β-glucuronidase in transformed cells: Methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant. Sci. 70, 133-40.
Moloney, M.M., Walker, J.M. and Sharma, K.K. (1989) High efficiency transformation of Brassica napususing Agrobacteriumvectors. Plant. Cell Rep. 8, 238-42.
Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473-97.
Odell, J.T., Nagy, F. and Chua, N.H. (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313, 810-2.
Pua, E.C., Mehra-Palta, A., Nagy, F. and Chua, N.H. (1987) Transgenic plants of Brassica napusL. Bio/Technology 5, 815-7.
Radke, S.E., Andrews, B.M., Moloney, M.M., Crouch, M.L., Kridl, J.C. and Knauf, V.C. (1988) Transformation of Brassica napusL. using Agrobacterium tumefaciens: developmentally regulated expression of an reintroduced napin gene. Theor. Appl. Genet. 75, 685-94.
Rasmussen, J. and Rasmussen, O.S. (1993) PEG mediated DNA uptake and transient GUS expression in carrot, rapeseed and soybean protoplasts. Plant Sci. 89, 199-207.
Schröder, M., Dixelius, C., Råhlén, L. and Glimelius, K. (1994) Transformation of Brassica napusby using the aadAgene as selectable marker and inheritance studies of the marker genes. Physiol. Plant. 92, 37-46.
Sjödin C. (1992) Brassicaceae, a plant family well suited for modern biotechnology. Acta. Agric. Scand. 42, 197-207.
Spangenberg, G., Neuhaus, G. and Schweiger, H.G. (1986) Expression of foreign genes in a higher plant cell after electrofusion-mediated cell reconstitution of a microinjected karyoplast and a cytoplast. Eur. J. Cell. Biol. 42, 236-8.
Stefanov, I., Sándor, F., Bögre, L., Pauk, J., Fehér, A. and Dutis, D. (1994) Differential activity of the mannopine synthase and the CaMV 35S promoters during development of transgenic rapeseed plants. Plant. Sci. 95, 175-86.
Velten, J. and Schell, J. (1985) Selection-expression plasmid vectors for use in genetic transformation of higher plants. Nucl. Acid Res. 13, 6981-98.
Waldron, C., Murphy, E.B., Roberts, J.L., Gustafson, G.D., Armour, S.L. and Malcolm, S.K. (1985) Resistance to hygromycin B. Plant Mol. Biol. 5, 103-8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Damgaard, O., Jensen, L.H. & Rasmussen, O.S. Agrobacterium tumefaciens-mediated transformation of Brassica napus winter cultivars. Transgenic Res 6, 279–288 (1997). https://doi.org/10.1023/A:1018458628218
Issue Date:
DOI: https://doi.org/10.1023/A:1018458628218