Abstract
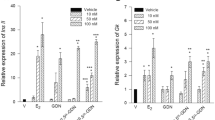
The pure antiestrogen ICI 182,780 has been shown to have antiprogestin activity in reporter gene constructs. Cell lines, naturally devoid of progesterone receptors (PR) were transfected with either the A or B forms of the human PR and a luciferase construct driven by a progesterone-response element (PRE). Because this system is an artificial one, our purpose was to determine whether these observations could be made in a human breast cancer cell line, naturally containing PR. We further evaluated the dose-response of ICI 182,780 and RU-486 (mifepristone) on PR and estrogen receptors (ER) in the presence of either progesterone, norgestrel or estradiol. These effects were measured using immunoassays for prostate-specific antigen (PSA) and human glandular kallikrein (hK2) and pS2. We found that ICI 182,780 blocked progesterone-stimulated PSA and hK2 production 100% at 10−5 M, which decreased significantly by 10−6 M. This inhibition did not occur when norgestrel was the progestin used. RU-486 showed 100% blockade for both progestins at all concentrations used. We concluded that the antiprogestin activity of ICI 182,780 exists for progesterone only. This weak antiprogestin activity may be unlikely to have significant clinical implications.
Similar content being viewed by others
References
Howell A, DeFriend D, Robertson J, Blamey R, Walton P: Response to a specific antioestrogen (ICI 182780) in tamoxifenresistant breast cancer. Lancet 345: 29–30, 1995
Wakeling AE, Dukes M, Bowler J: A potent specific pure antiestrogen with clinical potential. Cancer Res 51: 3867–3873, 1991
Fawell SE, White R, Hoare S, Sydenham M, Page M, Parker MG: Inhibition of estrogen receptor-DNA binding by the “pure” antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci USA 87: 6883–6887, 1990
Gibson MK, Nemmers LA, Beckman WC, Jr, Davis VL, Curtis SW, Korach KS: The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology 129: 2000–2010, 1991
Dauvois S, White R, Parker MG: The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci 106: 1377–1388, 1993
Howell A, Downey S, Anderson E: New endocrine therapies for breast cancer. Eur J Cancer 32A: 576–588, 1996
Kangas L, Nieminen AL, Blanco G, Gronroos M, Kallio S, Karjalainen A, Perila M, Sodervall M, Toivola R: A new triphenylethylene compound, Fc-1157a. II. Antitumor effects. Cancer Chemother Pharmacol 17: 109–113, 1986
Favoni RE, de Cupis A: Steroidal and nonsteroidal oestrogen antagonists in breast cancer: basic and clinical appraisal. Trends in Pharmaceutical Sciences 19: 406–415, 1998
Loser R, Seibel K, Roos W, Eppenberger U: In vivo and in vitro antiestrogenic action of 3-hydroxytamoxifen, tamoxifen and 4-hydroxytamoxifen: Eur J Cancer Clin Oncol 21: 985–990, 1985
Black LJ, Jones CD, Falcone JF: Antagonism of estrogen action with a new benzothiophene derived antiestrogen. Life Sci 32: 1031–1036, 1983
Toko T, Sugimoto Y, Matsuo K, Yamasaki R, Takeda S, Wierzba K, Asao T, Yamada Y: TAT-59, a new triphenylethylene derivative with antitumor activity against hormone-dependent tumors. Eur J Cancer 26: 397–404, 1990
Chander SK, McCague R, Luqmani Y, Newton C, Dowsett M, Jarman M, Coombes RC: Pyrrolidino-4-iodotamoxifen and 4-iodotamoxifen, new analogues of the antiestrogen tamoxifen for the treatment of breast cancer. Cancer Res 51: 5851–5858, 1991
Kraus WL, Weis KE, Katzenellenbogen BS: Inhibitory crosstalk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist-and antagonist-occupied progestin receptors. Mol Cell Biol 15: 1847–1857, 1995
Jeng MH, Langan-Fahey SM, Jordan VC: Estrogenic actions of RU486 in hormone-responsive MCF-7 human breast cancer cells. Endocrinology 132: 2622–2630, 1993
Nawaz Z, Stancel GM, Hyder SM: The pure antiestrogen ICI 182,780 inhibits progestin-induced transcription. Cancer Res 59: 372–376, 1999
Catherino WH, Jordan VC: Increasing the number of tandem estrogen response elements increases the estrogenic activity of a tamoxifen analogue. Cancer Lett 92: 39–47, 1995
Hall RE, Tilley WD, McPhaul MJ, Sutherland RL: Regulation of androgen receptor gene expression by steroids and retinoic acid in human breast-cancer cells. Int J Cancer 52: 778–784, 1992
Christopoulos TK, Diamandis EP: Enzymatically amplified time-resolved fluorescence immunoassay with terbium chelates. Anal Chem 64: 342–346, 1992
Yu H, Diamandis EP, Zarghami N, Grass L: Induction of prostate specific antigen production by steroids and tamoxifen in breast cancer cell lines. Breast Cancer Res Treat 32: 291–300, 1994
Black MH, Magklara A, Obiezu CV, Melegos DN, Diamandis EP: Development of an ultrasensitive immunoassay for human glandular kallikrein with no cross reactivity from prostate specific antigen. Clin Chem 45: 790–799, 1999
Phillips A, Demarest K, Hahn DW, Wong F, McGuire JL: Progestational and androgenic receptor binding affinities and in vivo activities of norgestimate and other progestins. Contraception 41: 399–410, 1990
Horowitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L: Nuclear receptor coactivators and corepressors. Mol Endocrinol 10: 1167–1177, 1996
Lange CA, Richer JK, Shen T, Horowitz KB: Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogenactivated protein kinase pathways. J Biol Chem 273: 31308–31316, 1998
Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horowitz KB: Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem 273: 31317–31326, 1998
Weaver CA, Springer PA, Katzenellenbogen BS: Regulation of pS2 gene expression by affinity labeling and reversibly binding estrogens and antiestrogens: comparison of effects on the native gene and on pS2-chloramphenicol acetyl transferase fusion genes transfected into MCF-7 human breast cancer cells. Mol Endocrinol 2: 936–945, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zand, R.S.R., Zand, R.S.R., Grass, L. et al. Is ICI 182,780 an antiprogestin in addition to being an antiestrogen?. Breast Cancer Res Treat 60, 1–8 (2000). https://doi.org/10.1023/A:1006334132303
Issue Date:
DOI: https://doi.org/10.1023/A:1006334132303