Abstract
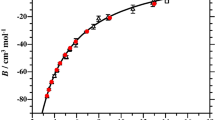
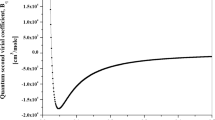
Second virial coefficients and transport properties of helium are presented based on a state-of-the-art interatomic potential which was constructed with the use of a multiproperty fit. The experimental potential employed to produce these properties accurately reproduces a wide range of bulk and microscopic data and agrees well with ab initio calculations which were not available at the time of its construction. Virial coefficients of 3He and 4He are presented from 2 to 600 K, and transport properties of pure 3He and 4He gases and 3He-4He mixtures are presented from 5 to 6000 K.
Similar content being viewed by others
References
M. Klein and H. J. M. Hanley, J. Chem. Phys. 53:4722 (1970).
R. A. Aziz and M. J. Slaman, Chem. Eng. Comm. 78:153 (1989).
J. H. Dymond and B. J. Alder, J. Chem. Phys. 51:309 (1969).
R. A. Aziz, F. R. W. McCourt, and C. C. K. Wong, Mol. Phys. 61:1487 (1987).
A. L. J. Burgmans, J. M. Farrar, and Y. T. Lee, J. Chem. Phys. 64:1345 (1976).
J. M. Farrar and Y. T. Lee, J. Chem. Phys. 56:5801 (1972).
R. Feltgen, H. Krist, K. A. Kohler, H. Pauly, and F. Torello, J. Chem. Phys. 76:2360 (1982).
W. Aufm Kampe, D. E. Oates, W. Schrader, and W. G. Bennewitz, Chem. Phys. Lett. 18:323 (1973).
R. Feltgen, H. Pauly, F. Torello, and H. Vehmeyer, Phys. Rev. Lett. 30:820 (1973).
R. A. Aziz and H. H. Chen, J. Chem. Phys. 67:5719 (1977).
R. Ahlrichs, P. Penco, and G. Scoles, Chem. Phys. 19:119 (1977).
R. A. Aziz and M. J. Slaman, Mol. Phys. 58:679 (1986).
R. A. Aziz and M. J. Slaman, Mol. Phys. 57:825 (1986).
R. A. Aziz and M. J. Slaman, Chem. Phys. 130:187 (1989).
D. A. Barrow and R. A. Aziz, J. Chem. Phys. 89:6189 (1988).
D. A. Barrow, M. J. Slaman, and R. A. Aziz, J. Chem. Phys. 91:6348 (1989).
D. M. Ceperley and H. J. Partridge, J. Chem. Phys. 84:820 (1986).
B. Liu and A. D. McLean, J. Chem. Phys. 91:2348 (1989).
J. H. van Lenthe, R. J. Vos, J. G. C. M. van Duijneveldt-van de Rijdt, and F. B. van Duijneveldt, Chem. Phys. Lett. 143:435 (1988).
F. B. van Duijneveldt, Private communication (1990).
A. Thakkar, J. Chem. Phys. 75:4496 (1981).
A. Koide, W. J. Meath, and A. R. Allnatt, J. Phys. Chem. 86:1222 (1982).
F. C. Matacotta, G. T. McConville, P. P. M. Steur, and M. Durieux, Metrologia 24:61 (1987).
G. T. McConville, Private communication (1987).
K. H. Berry, Metrologia 15:89 (1979).
R. C. Kemp, W. R. G. Kemp, and L. M. Besley, Metrologia 23:61 (1986/1987).
B. H. Gammon, J. Chem. Phys. 64:2556 (1976).
G. S. Kell, G. E. McLaurin, and E. Whalley, J. Chem. Phys. 68:2199 (1978).
J. C. Holste, M. Q. Watson, M. T. Bellomy, P. T. Eubank, and R. K. Hall, A.I.Ch.E. J. 26:954 (1980).
D. Gugan and W. D. Michel, Metrologia 16:149 (1980).
P. P. M. Steur, M. Durieux, and G. T. McConville, Metrologia 24:69 (1987).
E. Vogel, Ber. Bunsenges. Phys. Chem. 88:997 (1984).
E. Vogel, Z. Wilhelm-Pieck-Universitaet Rostock 33:34 (1984).
A. G. Clarke and E. B. Smith, J. Chem. Phys. 51:4156 (1969).
F. A. Guevara, B. B. McInteer, and W. E. Wageman, J. Chem. Phys. 12:2493 (1969).
J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids (Wiley, New York, 1954).
R. A. Aziz and A. R. Janzen, Metrologia 25:57 (1988).
R. A. Aziz and M. J. Slaman, Metrologia 27:211 (1990).
M. E. Boyd, S. Y. Larsen, and J. E. Kilpatrick, J. Chem. Phys. 50:4034 (1969).
J. Kestin, K. Knierim, E. A. Mason, B. Najafi, S. T. Ro, and M. Waldman, J. Chem. Phys. Ref. Data 13:229 (1984).
B. Najafi, E. A. Mason, and J. Kestin, Physica 119A:387 (1983).
J. Kestin, S. T. Ro, and W. A. Wakeham, Physica 58:165 (1972).
D. R. McLaughlin and H. F. Schaefer III, Chem. Phys. Lett 12:244 (1971).
R. A. Aziz, V. P. S. Nain, J. S. Carley, W. L. Taylor, and G. T. McConville, J. Chem. Phys. 70:4330 (1979).
P. K. Rol, as cited in R. A. Aziz, Inert. Gases, Chem. Phys. Ser. Vol. 34, M. L. Klein, ed. (Springer, Berlin, 1984).
J. Kestin, R. Paul, A. A. Clifford, and W. A. Wakeham, Physica 100A:349 (1980).
M. J. Assael, M. Dix, A. Lucas, and W. A. Wakeham, J. Chem. Soc. Faraday. Trans. I 77:439 (1981).
A. Acton and K. Kellner, Physica B 90:192 (1977).
J. W. Haarman, A.I.P. Conf. Proc. 11:193 (1973).
B. J. Jody, S. C. Saxena, V. P. S. Nain, and R. A. Aziz, Chem. Phys. 22:53 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Slaman, M.J., Aziz, R.A. Accurate transport properties and second virial coefficients for helium based on a state-of-the art interatomic potential. Int J Thermophys 12, 837–854 (1991). https://doi.org/10.1007/BF00502410
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00502410