Abstract
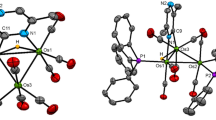
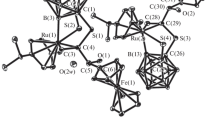
The title complex (complex1) was the first alkyne-substituted triruthenium dihydrido cluster to be reported and was characterized by spectroscopy as a triangular cluster with the alkyne parallel to a Ru-Ru edge. Recently, we have found that1 is a key intermediate in the homogeneous hydrogenation of diphenylacetylene catalyzed by tetrahedral Ru4 and FeRu3 clusters. Since the discovery of1, a great number of complexes with alkynes parallel to a cluster edge have been reported; at present this is the more common bonding mode for alkynes on trinuclear clusters. The structural features of1 allow a comparison with those of other ruthenium-containing derivatives and help to draw suggestions of the role of1 in hydrogenation catalysis.
Similar content being viewed by others
References
O. Gambino, E. Sappa, and G. Cetini (1972).J. Organomet. Chem. 44, 185.
A. J. P. Domingos, B. F. G. Johnson, and J. Lewis (1972).J. Organomet. Chem. 36, C 43.
A. J. Deeming, R. Ettorre, B. F. G. Johnson, and J. Lewis (1971).J. Chem. Soc. A 1797.
E. Sappa, O. Gambino, and G. Cetini (1972).J. Organomet. Chem. 35, 375.
G. Cetini, O. Gambino, E. Sappa, and M. Valle (1969).J. Organomet. Chem. 17, 437.
R. J. Goudsmit, B. F. G. Johnson, J. Lewis, P. R. Raithby, and M. J. Rosales (1983).J. Chem. Soc., Dalton Trans. 2257;
A. J. Deeming (1986).Adv. Organomet. Chem. 26, 1.
S. Aime, R. Gobetto, L. Milone, D. Osella, L. Violano, A. J. Arce, and Y. De Sanctis (1991).Organometallics 10, 2854.
M. Castiglioni, G. Gervasio, and E. Sappa (1981).Inorg. Chim. Acta 49, 217.
R. Amadelli, C. Bartocci, V. Carassiti, S. Aime, D. Osella, and L. Milone (1985).Gazz. Chim. It. 115, 337.
R. Giordano and E. Sappa (1993).J. Organomet. Chem. 448, 157.
E. Sappa, A. Tiripicchio, and P. Braunstein (1983).Chem. Rev. 83, 203;
E. Sappa, A. Tiripicchio, and P. Braunstein (1985).Coord. Chem. Rev. 65, 219.
J. F. Halet, J. Y. Saillard, R. Lissillour, M. J. McGlinchey, and G. Jaouen (1985).Inorg. Chem. 24, 218;
B. E. R. Schilling and R. Hoffmann (1979).J. Am. Chem. Soc. 101, 3456.
S. Rivomanana, G. Lavigne, N. Lugan, and J.-J. Bonnet (1991).Organometallics 10, 2285.
C. G. Pierpont (1977).Inorg. Chem. 16, 636.
S. A. MacLaughlin, N. J. Taylor, and A. J. Carty (1984).Organometallics 3, 392.
E. Sappa, A. M. Manotti Lanfredi, and A. Tiripicchio (1981).J. Organomet. Chem. 221, 93.
P. Braunstein, J. Rosé, and O. Bars (1983).J. Organomet. Chem. 252, C 101.
F. W. B. Einstein, K. G. Tyers, A. S. Tracey, and D. Sutton (1986).Inorg. Chem. 25, 1631.
E. Rosenberg, J. Bracker Novak, R. W. Gellert, S. Aime, R. Gobetto, and D. Osella (1989).J. Organomet. Chem. 365, 163.
S. A. MacLaughlin, J. P. Johnson, N. J. Taylor, A. J. Carty, and E. Sappa (1983).Organometallics 2, 352.
E. Sappa, A. Tiripicchio, and M. Tiripicchio Camellini (1981).J. Organomet. Chem. 213, 175.
R. P. Dodge and V. Shomaker (1965).J. Organomet. Chem. 3, 274.
B. F. G. Johnson, J. Lewis, B. E. Reichert, K. T. Schorpp, and G. M. Sheldrick (1977).J. Chem. Soc., Dalton Trans. 1417.
J. Wang, M. Sabat, L. J. Lyons, and D. F. Shriver (1991).Inorg. Chem. 30, 382.
J. R. Fox, W. L. Gladfelter, G. L. Geoffroy, I. Tavanaiepour, S. Abdel-Mequid, and V. W. Day (1981).Inorg. Chem. 20, 3230.
S. A. MacLaughlin, D. Nucciarone, and A. J. Carty, in J. G. Verkade and L. D. Quinn (eds.),Phosphorus-31 NMR Spectroscopy in Structural Analysis; Organic Compounds and Metal Complexes (VCH Publishers, New York, 1987), Chap. 16.
R. Poilblanc (1982).Inorg. Chim. Acta 62, 75.
M. Castiglioni, R. Giordano, and E. Sappa (1989).J. Organomet. Chem. 369, 419.
R. D. Adams, Z. Li, P. Swepston, W. Wu, and J. Yamamoto (1992).J. Am. Chem. Soc. 114, 10657.
N. Walker and D. Stuart (1983).Acta Crystallogr., Sect. A 39, 158;
F. Ugozzoli (1987).Comput. Chem. 11, 109.
International Tables for X-Ray Crystallography (Kynoch Press, Birmingham, England, 1974), Vol. IV.
G. M. Sheldrick, SHELX-76 Program for Crystal Structure Determination (University of Cambridge, England, 1976); SHELXS-86 Program for the Solution of Crystal Structures (University of Göttingen, 1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cauzzi, D., Giordano, R., Sappa, E. et al. The first ruthenium-alkyne-dihydride reported is now recognized as an intermediate in the homogeneous hydrogenation of diphenylacetylene: Crystal structure of (μ-H)2Ru3(CO)9(μ3-η2-∥-C2Ph2). J Clust Sci 4, 279–296 (1993). https://doi.org/10.1007/BF00703744
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00703744