Abstract
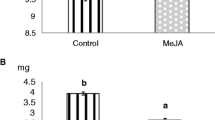
Some plant defenses are known to be rapidly induced following attack by phytophagous insects. Plant-produced insect molting hormones, termed phytoecdysteroids, are believed to aid plant resistance; however, their dynamics are poorly understood. Using spinach (Spinacia oleracea) as a model system, we examined the inducibility of phytoecdysteroids, primarily 20-hydroxyecdysone (20E), in an effort to characterize potential interactions with herbivorous insects. Rapid phytochemical induction was investigated using damage treatments and applications of defense-related plant-signal analogs, specifically methyl jasmonate (MJ) and methyl salicylate (MSA). Within two days, mechanically damaged roots exhibited two to three fold increases in phytoecdysteroid concentrations. Four days after root damage, small increases in shoot levels were also detectable. Unlike roots, foliar 20E concentrations were unaltered over a range of shoot treatments including insect herbivory (Spodoptera exigua), mechanical damage, and MJ applications. Additions of MJ (12.5–50 μg/liter) to the root systems of hydroponically grown plants stimulated accumulations of root phytoecdysteroids in a dose-dependent manner, similar in magnitude to the response induced by root damage. Under identical conditions, MSA did not affect the accumulation of 20E when added to the hydroponic solutions of undamaged plants. Moreover, MSA inhibited the induction of 20E in wounded roots, but did not interfere with the action of applied MJ. In contrast to mechanical damage, roots did not induce 20E levels when challenged with two different fungal pathogens (Pythium aphanidermatum and Phytophthora capsici).We propose that wound-induced accumulations of 20E are generated in the roots, signaled via endogenous jasmonates, and may confer enhanced resistance against subterranean herbivorous insects.
Similar content being viewed by others
REFERENCES
Adler, J. H., and Grebenok, R. J. 1997. Occurrence, biosynthesis, and putative role of ecdysteroids in plants, pp. 181–192, in E. J. Parish and W. D. Nes (eds.). Biochemistry and Function of Sterols. CRC Press, Boca Raton, Florida.
Alborn, H. T., Turlings, T. C. J., Jones, T. H., Stenhagen, G., Loughrin, J. H., and Tumlinson, J. H. 1997. An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949.
Arnault, C., and Slama, K. 1986. Dietary effects of phytoecdysones in the leek-moth, Acrolepiopsis assectella Zell. (Lepidoptera: Acrolepiidae). J. Chem. Ecol. 12:1979–1986.
Baldwin, I. T. 1990. Herbivory simulations in ecological research. Tree 5:91–93.
Baldwin, I. T. 1994. Chemical changes rapidly induced by folivory, pp. 1–23, in E. A. Bernays (ed.). Insect-Plant Interactions, Vol. 5. CRC Press, Boca Raton, Florida.
Baldwin, I. T., Schmelz, E. A., and Ohnmeiss, T. E. 1994. Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris. J. Chem. Ecol. 20:2139–2157.
Baldwin, I. T., Schmelz, E. A., and Zhang, Z.-P. 1996. Effects of octadecanoid metabolites and inhibitors on induced nicotine accumulation in Nicotiana sylvestris. J. Chem. Ecol. 22:61–74.
Baldwin, I. T., Zhang, Z.-P., Diab, N., Ohnmeiss, T. E., McCloud, E. S., Lynds, G. L., and Schmelz, E. A. 1997. Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta 201:397–404.
Bergamasco, R., and Horn, D. H. S. 1983. Distribution and role of insect hormones in plants, pp. 627–654, in R. G. H. Downer, and H. Laufer (eds.). Invertebrate Endocrinology, Vol. 1: Endocrinology of Insects. Liss, New York.
Blackford, M., and Dinan, L. 1997. The effects of ingested 20–hydroxyecdysone on the larvae of Aglais urticae, Inachis io, Cynthia cardui (Lepidoptera: Nymphalidae) and Tyria jacobaeae (Lepidoptera: Artiidae). J. Insect Physiol. 43:315–327.
Blackford, M., Clarke, B. S., and Dinan, L. 1996. Tolerance of the Egyptian cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae) to ingested phytoecdysteroids. J. Insect Physiol. 42:931–936.
Brown, V. K., and Gange, A. C. 1990. Insect herbivory below ground. Adv. Ecol. Res. 20:1–58.
Carlisle, D. B., Osborne, D. J., Ellis, P. E., and Moorhouse, J. E. 1963. Recpirocal effects of insect and plant growth substances. Nature 200:1230.
Choi, D., Bostock, R. M., Avdiushko, S., and Hildebrand, D. F. 1994. Lipid-derived signals that discriminate wound and pathogen responsive isoprenoid pathways in plants: Methyl jasmonate and the fungal elicitor arachidonic acid induce different 3–hydroxy-3–methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids in Solanum tuberosum. L. Proc. Natl. Acad. Sci. U.S.A. 91:2329–2333.
Cooke, D. A., and Dewar, A. M. 1992. Pests of Chenopodiaceous crops, pp. 28–73, in R. G. McKinlay (ed.). Vegetable Crop Pests. Macmillian Press, Hong Kong.
Creelman, R. A., Tierney, M. L., and Mullet, J. E. 1992. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc. Natl. Acad. Sci. U.S.A. 89:4938–4941.
Dreier, S. I., and Towers, G. H. N. 1988. Activity of ecdysterone in selected plant growth assays. J. Plant Physiol. 132:509–512.
Enyedi, A. J., Yalpani, N., Silverman, P., and Raskin, I. 1992. Signal molecules in systemic plant resistance to pathogens and pests. Cell 70:879–886.
Farmer, E. E. 1994. Fatty acid signaling in plants and their associated microorganisms. Plant Mol. Biol. 26:1423–1427.
Farmer, E. E., and Ryan, C. A. 1990. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. U.S.A. 87:7713–7716.
Farmer, E. E., and Ryan, C. A. 1992. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase. Plant Cell 4:129–134.
Farmer, E. E., Johnson, R. R., and Ryan, C. A. 1992. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol. 98:995–1002.
Felippe, G. M. 1980. Insect growth hormones and their effects on some plants. Cienc. Cult. (San Paulo) 32:1384–1390.
Galbraith, M. N., and Horn, D. H. S. 1966. An insect-moulting hormone from a plant. J. Chem. Soc. Chem. Commun. 1966:905–906.
Grebenok, R. J., and Adler, J. H. 1991. Ecdysteroid distribution during development of spinach. Phytochemistry 30:2905–2910.
Grebenok, R. J., and Adler, J. H. 1993. Ecdysteroid biosynthesis during the ontogeny of spinach leaves. Phytochemistry 33:341–347.
Grebenok, R. J., Ripa, P. V., and Adler, J. H. 1991. Occurrence and levels of ecdysteroids in spinach. Lipids 26:666–668.
Gundlach, H., MÜller, M. J., Kutchan, T. M., and Zenk, M. H. 1992. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl. Acad. Sci. U.S.A. 89:2389–2393.
Hamberg, M., and Gardner, H. W. 1992. Oxylipin pathway to jasmonates: biochemistry and biological significance. Biochim. Biophys. Acta 1165:1–18.
Heftman, E. 1975. Steroid hormones in plants. Lloydia 38:195–209.
Hendrix, S. D., and Jones, R. L. 1972. The activity of β-ecdysone in four gibberellin bioassays. Plant Physiol. 50:199–200.
Hoagland, D. R., and Arnon, D. I. 1950. The water culture method for growing plants without soil. California Agriculture Station Circular 347.
Huang, H-M., Johanning, G. L., and O'Dell, B. L. 1986. Phenolic acid content of food plants and possible nutritional implications. J. Agric. Food Chem. 34:48–51.
Huber, R., and Hoppe, W. 1965. Zur Chemie des Ecdysones. VII. Die Kristall-und Molekülstrukturanalyse des Insektenverpuppungs hormons Ecdyson mit der automatisierten Faltmolecülemthode. Chem. Ber. 98:2403–2424.
Jacobs, W. P., and Suthers, H. B. 1971. The culture of apical buds of Xanthium and their use as a bioassay for flowering activity of ecdysterone. Am. J. Bot. 58:836–843.
Jones, C. G., and Firn, R. D. 1978. The role of phytoecdysteroids in bracken fern, Pteridium aquilinum (L.) Kuhn as a defense against phytophagous insect attack. J. Chem. Ecol. 4:117–138.
Jones, F. G. W., and Dunning, R. A. 1969. Sugar Beet Pests. Bulletin 162, 2nd ed. HMSO, London.
Karban, R., and Myers, J. H. 1989. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 20:331–348.
Klessig, D. F., and Malamy, J. 1994. The salicylic acid signal in plants. Plant Mol. Biol. 26:1439–1458.
Koolman, J. 1989. Ecdysone: From Chemistry to Mode of Action. Thieme Medical Publishers, New York, 482 pp.
Kubo, I., and Klocke, J. A. 1983. Isolation of phytoecdysones as insect ecdysis inhibitors and feeding deterrents, pp. 329–346, in P. A. Hedin (ed.). Plant Resistance to Insects, ACS Symposium Series. American Chemical Society, Washington, DC.
Kubo, I., Klocke, J. A., and Asano, S. 1983. Effects of ingested phytoecdysteroids on the growth and development of two larvae. J. Insect. Physiol. 29:307–316.
Lafont, R. 1997. Ecdysteroids and related molecules in animals and plants. Arch. Insect Biochem. Phys. 35:3–20.
Lafont, R., and Horn, D. S. 1989. Phytoecdysteroids: Structures and occurrence, pp. 39–64, in J. Koolman (ed.). Ecdysone: From Chemistry to Mode of Action. Thieme Medical Publishers, New York.
Lafont, R., Bouthier, A., and Wilson, I. D. 1991. Phytoecdysteroids: Structures, occurrence, biosynthesis and possible significance, pp. 197–214, in I. Hrdy (ed.). Insect Chemical Ecology. Academia, Prague, and SPB Academic Publishers, The Hague.
Lange, W. H. 1987. Insect pests of sugar beet. Annu. Rev. Entomol. 32:341–360.
Larsson, M. 1992. Soilborne root pathogens of spinach in southern Sweden. Vaextskyddsrapporter Avhandlingar 24. Uppsala, Sweden.
Libert, B., and Franceschi, V. R. 1987. Oxalate in crop plants. J. Agric. Food. Chem. 35:926–938.
MachÁckovÁ, I., VÁgner, M., and SlÁma, K. 1995. Comparison between the effects of 20–hydroxyecdysone and phytohormones on growth and development in plants. Eur. J. Entomol. 92:309–316.
E. MelÉ J. Messeguer J. Gabarra J. TomÁs J. Coll F. Camps (1992) ArticleTitleIn vitro bioassay for the effect of Ajuga reptans phytoecdysteroids on Trialeurodes vaporariorum larval development. Entomol. Exp. Appl. 62 163–168
Mizukami, H., Tabira, Y., and Ellis, B. E. 1993. Methyl jasmonate-induced rosemarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant Cell Rep. 12:706–709.
Modde, J-F., Lafont., R., and Hoffmann, J. A. 1984. Ecdysone metabolism in Locusta migratoria larvae and adults. Invert. Reprod. Dev. 7:161–183.
Morgan, E. D., and Macro, M. P. 1990. Advances in techniques for ecdysteroid analysis. Invert. Reprod. Dev. 18:55–66.
Mueller, M. J., Brodschelm, W., Spannagl, E., and Zenk, M. H. 1993. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc. Natl. Acad. Sci. U.S.A. 90:7490–7494.
Nakanishi, K., Koreeda, M., Dasaka, S., Chang, M. L., and Hsu, H. Y. 1966. Insect hormones. I. The structure of ponasterone A, an insect moulting hormone from the leaves of Podocarpus nakaii Hay. J. Chem. Soc. Chem. Commun. 1966:915–917.
Ohnmeiss, T. E., and Baldwin, I. T. 1994. The allometry of nitrogen allocation to growth and an inducible defense under nitrogen-limited growth. Ecology 75:995–1002.
PeÑa-CortÉs, H., Albrecht, T., PRAT, S., E. W., and Willmitzer, L. 1993. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191:123–128.
PeÑa-CortÉs, H., Fisahn, J., and Willmitzer, L. 1995. Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc. Natl. Acad. Sci. U.S.A. 92:4106–4113.
Rees, H. H. 1989. Zooecdysteroids: structures and occurrence, pp. 28–38, in J. Koolman (ed.). Ecdysone: From Chemistry to Mode of Action. Thieme Medical Publishers, New York.
Robbins, W. E., Kaplanis, J. N., Thompson, M. J., Shortino, T. J., and Joyner, S. C. 1970. Ecdysones and synthetic analogs: Molting hormone activity and inhibitive effects on insect growth, metamorphosis, and reproduction. Steroids 16:105–125.
Rubatzky, V. E., and Yamaguchi, M. 1997. World Vegetables: Principles, Production, and Nutritive Values, 2nd ed. Chapman and Hall, New York.
Ryan, C. A. 1992. The search for the proteinase inhibitor-inducing factor, PIIF. Plant Mol. Biol. 19:123–133.
Ryan, C. A., and Jagendorf, A. 1995. Self defense by plants. Proc. Natl. Acad. Sci. U.S.A. 92:4075.
Silvertown, J., and Gordon, D. M. 1989. A framework for plant behavior. Annu. Rev. Ecol. Syst. 20:349–366.
Singh, P., and Russell, G. B. 1980. The dietary effects of 20–hydroxyecdysone on the development of housefly. J. Insect. Physiol. 26:139–142.
SlÁma, K. 1979. Insect hormones and antihormones in plants, pp. 683–700, in G. A. Rosenthal and D. H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York.
Stanghellini, M. E., Rasmussen, S. L., Kim, D. H., and Rorabaugh, P. A. 1996. Efficacy of nonionic surfactants in the control of zoospore spread of Pythium aphanidermatum in a recirculating hydroponic system. Plant Dis. 80:422–428.
Tallamy, D. W., and Raupp, M. J. 1991. Phytochemical Induction by Herbivores. John Wiley & Sons, New York, 932 pp.
Tanaka, Y., Asaoka, K., and Takeda, S. 1994. Different feeding and gustatory responses to ecdysone and 20–hydroxyecdysone by larvae of the silkworm, Bombyx mori. J. Chem. Ecol. 20:125–133.
TomÁs, J., Camps, F., Cool, J., MelÉ, E., and Messeguer, J. 1993. Phytoecdysteroid production by Ajuga reptans tissue cultures. Phytochemistry 32:317–324.
Tschesche, R., Rehkamper, H., and Wulff, G. 1969. Über die saponine des spinats (Spinacia oleracea L.). Liebigs Ann. Chem. 726:125–135.
Ward, E. R., Uknes, S. J., Williams, S. C., Dincher, S. S., Wiederhold, D. L., Alexander, D. C., Ahl-Goy, P., MÉtraux, J-P., and Ryals, J. A. 1991. Coordinate gene activity in response to agents that induce systemic required resistance. Plant Cell 3:1085–1094.
Whitney, E. D., and Duffus, J. E. 1986. Compendium of Beet Diseases and Insects. APS Press, St. Paul, Minnesota, 76 pp.
Wilkinson, L., and Hill, M. 1994a. Systat for DOS: Using SYSTAT. Version 6. SYSTAT, Inc., Evanston, Illinois, 872 pp.
Wilkinson, L., and Hill, M. 1994b. Systat for DOS: Advanced Applications. Version 6. SYSTAT, Inc., Evanston, Illinois, 901 pp.
Zangerl, A. R., and Rutledge, C. E. 1996. The probability of attack and patterns of constitutive and induced defense: A test of optimal defense theory. Am. Nat. 147:599–608.
Zar, J. H. 1996. Biostatistical Analysis, 3rd ed. Prentice-Hall, Upper Saddle River, New Jersey, 662 pp.
Zhang, M., and Kubo, I. 1993. Metabolic fate of ecdysteroids in larval Bombyx mori and Heliothis virescens. Insect Biochem. Mol. Biol. 23:831–843.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmelz, E.A., Grebenok, R.J., Galbraith, D.W. et al. Damage-Induced Accumulation of Phytoecdysteroids in Spinach: A Rapid Root Response Involving the Octadecanoic Acid Pathway. J Chem Ecol 24, 339–360 (1998). https://doi.org/10.1023/A:1022588610232
Issue Date:
DOI: https://doi.org/10.1023/A:1022588610232