Abstract
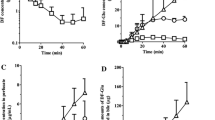
The influence of enzymic distribution on lidocaine metabolism was investigated in the once-through perfused rat liver preparation. Low input concentrations of14C-lidocaine (1–2 μM) and preformed monoethylglycine xylidide (MEGX; 2.3–2.8 μM) were delivered by normal and retrograde flow directions to the liver preparations at 10 ml/min per liver. Upon reversal of normal to retrograde delivery of lidocaine, the rates at which lidocaine, MEGX, and glycine xylidide (GX) left the liver almost doubled, whereas the rates of appearance of (total) hydroxylated lidocaine and MEGX in bile and perfusate increased to lesser extents. Upon reversal of normal to retrograde delivery of preformed MEGX, the rates of appearance of MEGX and GX were virtually unchanged. Computer simulations on lidocaine and preformed MEGX metabolism were performed on both evenly distributed (“parallel tube” model) and enzyme-distributed systems. An even or parallel distribution of N-deethylation and hydroxylation activities for lidocaine metabolism failed to predict the observed increased hepatic availability of lidocaine. Rather, the distribution of a low-affinity, high-capacity N-deethylation system anterior to a high-affinity, lowcapacity hydroxylation system for lidocaine metabolism adequately predicted the increased hepatic availability of lidocaine. Further extension of these consistent enzyme-distributed models on the metabolism of lidocaine metabolites suggests that the N-deethylation and hydroxylation activities for the metabolism of lidocaine, MEGX, 3-hydroxyidocaine, and 3-hydroxy MEGX are not identically distributed. When these enzymedistributed models were appraised with reference to the “parallel tube” and “wellstirred” models of hepatic drug clearance, predictions from these.enzymedistributed models proved to be superior to the “parallel tube” and “well-stirred” models for the present data on lidocaine metabolites with normal and retrograde perfusions. Previously published data on lidocaine and MEGX metabolism after inputting 4 μg/ml (17 μM) lidocaine at flow rates of 10, 12, 14, and 16 ml/min were reexamined with respect to the adequacy of these enzyme-distributed models. They were found to be superior to the evenly-distributed or “parallel tube” model in predicting hepatic availability of lidocaine and the rate of appearance of MEGX. However, the enzyme-distributed systems were not as consistent as the “well-stirred” model in predicting lidocaine hepatic availability in these flow experiments.
Similar content being viewed by others
References
R. N. Boyes, D. B. Scott, P. J. Jebson, M. J. Goodman, and D. G. Julian. Pharmacokinetics of lidocaine in man.Clin. Pharmacol. Ther. 12:105–114 (1970).
E. S. Munson, R. W. Martucci, and I. H. Wagman. Bupivacaine and lignocaine induced seizures in Rhesus monkeys.J. Anesth. 44:1025–1028 (1972).
M. A. Nevins. Re-evaluating the use of lidocaine. Toxic and metabolic effects, sinus node depression and paradoxical cardiac influences are potential hazards and limitations.Geriatrics 28:48–51 (1973).
H. J. Pfeifer, D. J. Greenblatt, and J. Koch-Weser. Clinical use and toxicity of intravenous lidocaine. A report from the Boston Collaborative Drug Surveillance Program.Am. Heart J. 92:168–173 (1976).
M. G. Wyman, D. Lalka, L. Hammersmith, D. S. Cannom, and B. N. Goldreyer. Multiple bolus technique for lidocaine administration during the first hours of an acute myocardial infarction.Am. J. Cardiol. 41:313 (1978).
W. W. Stargel, D. G. Shand, P. A. Routledge, A. Barchowsky, and G. S. Wagner. Clinical comparison of rapid infusion and multiple injection methods for lidocaine loading.Am. Heart J. 102:872–876 (1981).
F. G. McMahon and L. A. Woods. Estimation of metabolism of lidocaine (Xylocaine), alpha-dimethylamino-2,6-acetoxylidide.Fed. Proc. 10:321 (1951).
G. D. Breck and W. F. Trager. Oxidative N-dealkylation. A Mannich intermediate in the formation of a new metabolite of lidocaine in man.Science 173:545–546 (1971).
J. Thomson and P. Meffin. Aromatic hydroxylation of lidocaine and mepivacaine in rats and humans.J. Med. Chem. 15:1046–1049 (1972).
J. M. Strong, M. Parker, and A. J. Atkinson, Jr. Identification of glycine xylidide in patients treated with intravenous lidocaine.Clin. Pharmacol. Ther. 14:67–72 (1972).
K. K. Adjepon-Yamoah and L. F. Prescott. Lignocaine metabolism in man.Br. J. Pharmacol. 47:672–673 (1973).
F. G. McMahon and L. A. Woods. Further studies on the metabolism of lidocaine (xylocaine) in the dog.J. Pharmacol Exp. Ther. 103:354 (1951).
I. C. Geddes. The use of C14 to establish the metabolism of lignocaine.Anesthesia 13:200 (1958).
G. Hollunger. On the metabolism of lidocaine. II. Biotransformation of lidocaine.Acta Pharmacol. Toxicol. 17:365–373 (1960).
J. B. Keenaghen and R. N. Boyes. The tissue distribution, metabolism, and excretion of lidocaine in rats, guinea pigs, dogs and man.J. Pharmacol. Exp. Ther. 180:459–463 (1972).
C. A. DiFazio and R. E. Brown. Lidocaine metabolism in normal and phenobarbital pretreated dogs.Anesthesiology 36:238–243 (1972).
G. Hollunger. Solubilisation and purification of an amide-hydrolysing microsomal enzyme.Acta Pharmacol. Toxicol. 17:374–383 (1960).
C. von Bahr, I. Hedlund, B. Karlen, D. Backtrom, and H. Grasdalen. Evidence for two catalytically different binding sites of liver microsomal cytochrome P-450: Importance for species and sex differences in oxidation pattern of lidocaine.Acta Pharmacol. Toxicol. 41:39–48 (1977).
E. S. Munson, R. W. Martucci, and I. H. Wagman. Bupivacaine and lignocaine induced seizures in Rhesus monkeys.J. Anesih. 44:1025–1028 (1972).
E. S. Smith and B. R. Duce. The acute antiarrhythmic and toxic effects in mice and dogs of 2-ethylamino-2′,6-acetoxylidide (L-86), a metabolite of lidocaine.J. Pharmacol. Exp. Ther. 179:580–585 (1971).
J. Blumer, J. M. Strong, and A. J. Atkinson, Jr. The convulsant potency of lidocaine and its N-dealkylated metabolites.J. Pharmacol Exp. Ther. 186:31–36 (1973).
R. G. Burner, C. A. DiFazio, M. J. Peach, K. A. Petrie, and M. J. Silvester. Antiarrhythmic effects of lidocaine metabolites.Am. Heart. J. 88:765–769 (1974).
J. M. Strong, D. E. Mayfield, A. J. Atkinson, Jr., B. C. Burris, F. Raymon, and L. T. Webster, Jr. Pharmacologic activity, metabolism and pharmacokinetics of glycine-xylidide.Clin. Pharmacol. Ther. 17:184–194 (1974).
J. LeLorier, D. Grenon, Y. Latour, and G. Caille. Pharmacokinetics of lidocaine after prolonged intravenous infusions in uncomplicated myocardiai infarction.Ann. Intern. Med. 87:700–702 (1977).
D. S. Fredrick and R. B. Boersma. Lidocaine infusions: Effect of duration and method of discontinuation of recurrence of arrhythmias and pharmacokinetic variables.Am. J. Hosp. Pharm. 36:778–781 (1979).
J. LeLorier, R. Mosian, J. Gagne, and G. Caille. Effect of duration of infusion on the disposition of lidocaine in dogs.J. Pharmacol. Exp. Ther. 203:507–511 (1977).
N. Vicuna, D. Lalka, S. R. Burrow, A. J. McLean, P. du Souich, and J. L. McNay. Dose-dependent pharmacokinetic behavior of lidocaine in the conscious dog.Res. Commun. Chem. Pathol. Pharmacol. 22:485–491 (1978).
M. S. Lennard, G. T. Tucker, and H. F. Woods. Time-dependent kinetics of lignocaine in the isolated perfused rat liver.J. Pharmacokin. Biopharm. 11:165–182 (1983).
K. S. Pang and M. Rowland. Hepatic clearance of drugs. II. Experimental evidence for the acceptance of the “well-stirred” model over the “parallel tube” model using lidocaine in the perfused rat liverin situ preparation.J. Pharmacokin. Biopharm. 5:655–680 (1977).
T. Suzuki, S. Fujita, and R. Kawai. Precursor-metabolite interaction in the metabolism of lidocaine.J. Pharm. Sci. 73:136–138 (1984).
R. Kawai, J. Tatsuno, S. Fujita, and T. Suzuki. Lidocaine-lidocaine metabolite interactions in their metabolism.J. Pharmacokin. Biodyn. 6:s-79 (1983).
K. S. Pang and M. Rowland. Hepatic clearance of drugs. III. Additional experimental evidence for the acceptance of the “well-stirred” model using metabolite (MEGX) data generated from lidocaine under varying hepatic blood flows in the rat liver perfusedin situ preparation.J. Pharmacokin. Biopharm. 5:681–699 (1977).
K. S. Pang and J. A. Terrell. Retrograde perfusion to probe the heterogeneous distribution of hepatic drug metabolizing enzymes in rats.J. Pharmacol Exp. Ther. 216:339–346 (1981).
P. K. Narang, W. G. Crouthamel, N. H. Carliner, and M. L. Fisher. Lidocaine and its active metabolites.Clin. Pharmacol. Ther. 24:654–662 (1978).
S. D. Nelson, W. A. Garland, G. D. Breck, and W. F. Trager. Quantitation of lidocaine and several metabolites utilizing chemical-ionization mass spectroscopy and stable isotope labelling.J. Pharm. Sci. 66:1180–1190 (1977).
G. Knott and D. Reece.MLAB, An On Line Modeling Laboratory, 7th ed., Division of Computer Research and Technology, National Institutes of Health, Bethesda, Maryland, 1977.
K. S. Pang, H. Koster, I. C. M. Halsema, E. Scholtens, G. J. Mulder, and R. N. Stillwell. Normal and retrograde perfusion to probe the zonal distribution of sulfation and glucuronidation activities of harmol in the perfused rat liver preparation.J. Pharmacol. Exp. Ther. 224:647–653 (1983).
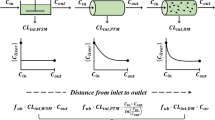
K. S. Pang and R. N. Stillwell. An understanding of the role of enzyme localization of the liver on metabolite kinetics: A computer simulation.J. Pharmacokin. Biopharm. 11:451–467 (1983).
J. Baron, J. A. Redick, and F. P. Guengerich. Immunohistochemical localizations of cytochrome P-450 in the rat liver.Life Sci. 23:2627–2632 (1978).
D. G. Shand, D. M. Kornhauser, and G. R. Wilkinson. Effects of route of administration and blood flow on hepatic drug elimination.J. Pharmacol. Exp. Ther. 195:424–432 (1975).
M. Rowland, L. Z. Benet, and G. G. Graham. Clearance concepts in pharmacokinetics.J. Pharmacokin. Biopharm. 1:123–136 (1973).
K. Winkler, S. Keiding, and N. Tygstrup. Clearance as a quantitative measure of liver function. In P. Paumgartner and R. Presig (eds.),The Liver: Quantitative Aspects of Structure and Function, Karger, Basel, 1973, pp. 144–155.
K. S. Pang. Hepatic Clearance of Drugs. Ph.D. thesis, University of California at San Francisco, 1976.
C. A. Goresky. A linear method for determining liver sinusoidal and extravascular volumes.Am. J. Physiol. 204:626–640 (1963).
M. S. Roberts and M. Rowland. Communication. Hepatic elimination—Dispersion model.74: 585–587 (1985).
L. Bass, P. Robinson, and A. J. Bracken. Hepatic elimination of flowing substrates: The distributed model.J. Theor. Biol. 72:161–184 (1978).
B. A. Luxon and E. L. Forker. Hepatic transport kinetics and plasma disappearance curves: Distributed modeling versus conventional approach.Am. J. Physiol. 235:E648-E660 (1978).
E. L. Forker and B. A. Luxon. How to measure first-order hepatic transfer coefficients by distributed modeling of a recirculating rat liver perfusion system.Am. J. Physiol. 243:G518-G531 (1982).
Y. Sawada, Y. Sugiyama, Y. Miyamoto, T. Iga, and M. Hanano. Hepatic clearance model: Comparison among the distributed, parallel-tube and well-stirred models.Chem. Pharm. Bull. 33:319–326 (1985).
Author information
Authors and Affiliations
Additional information
This work was supported in part by U.S. HHS grant GM-27323 and a Research Career Development Award from the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases (AM-01028) as well as grants from the Canadian Medical Research Council (development grants DG-262, 263, and 264) (K.S.P.).
Rights and permissions
About this article
Cite this article
Pang, K.S., Terrell, J.A., Nelson, S.D. et al. An enzyme-distributed system for lidocaine metabolism in the perfused rat liver preparation. Journal of Pharmacokinetics and Biopharmaceutics 14, 107–130 (1986). https://doi.org/10.1007/BF01065257
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01065257