Abstract
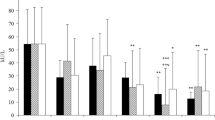
S. typhimurium infection is associated with neutrophil infiltration within the intestinal mucosa. Neutrophil activation provides a major source of reactive oxygen species (ROS). The mucosal pathology of S. typhimurium infection may be in part due to the excessive production of these reactive species. This study was carried out to investigate if ROS play a role in mediating the changes in the structural components and functional properties of brush border membrane (BBM) in rats during S. typhimurium infection. This was done by determining the changes in the BBM extent of lipid peroxidation and absorptive function. A significant increase in the extent of lipid peroxidation of BBM during S. typhimurium infection was observed as judged by malondialdehyde (MDA) and conjugated diene formation and depletion of α-tocopherol and protein associated thiol groups. A significant decrease in the BBMV (brush border membrane vesicle) transport of amino acids was also observed. However there was no change in the transport of D-glucose. The decrease in amino acid transport further led to a significant decrease in the enterocyte level of protein synthesis. Exposure of BBMV to a free radical donor, cumene hydroperoxide, also led to an increase in the extent of lipid peroxidation and a decrease in the amino acid transport. Possibly ROS might play a significant role in mediating the mucosal damage during S. typhimurium infection.
Similar content being viewed by others
References
Wallis TS, Hauker RJH, Candy DCA, Qi GM, Clarke GJ, Worton KJ, Osborne MP, Stephen J: Quantification of the leukocyte influx into rabbit ileal loops induced by strains of Salmonella typhimurium of different virulence. J Med Microbiol 30: 149–156, 1989
Wallis TS, Vaughan ATM, Clarke GJ, Qi GM, Worton KJ, Candy DCA, Osborne MP, Stephen, J: The role of leukocytes in the induction of fluid secretion by Salmonella typhimurium. J Med Microbiol 31: 27–35, 1990
McCormick BA, Miller SI, Cornes D, Madara JL: Transepithelial signalling to neutrophils by Salmonellae: A novel virulence mechanism for gastroenteritis. Infect Immun 63: 2302–2309, 1995
Aruoma OJ, Halliwell B, Gajewski E, Dizdaraglu M: Copper-iron dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J 273: 2601–2604, 1991
Smith S, Grisham M, Manci E, Granger N, Kvietys P: Gastric mucosal injury in the rat. Gastroenterology 92: 950–956, 1981
Oshitani N, Kitano A, Okabe H, Nakamura S, Matsumoto T, Kobayashi K: Location of superoxide anion generation in human colonic mucosa obtained by biopsy. Gut 34: 936–938, 1993
Pryor WA: Free radicals and lipid peroxidation: What they are and how they got that way. In: B Frei (ed). Natural Antioxidants in Human Health and Disease. Orlando, FL: Academic Press, 1994, 1–24
Nalini S, Ibrahim SA, Balasubramanian KA: Effect of oxidant exposure on monkey intestinal brush-border membrane. Biochim Biophys Acta 1147: 169–176, 1993
Jourdheuil D, Vaananen P, Meddings JB: Lipid peroxidation of the brush border membrane: membrane physical properties and glucose transport. Am J Physiol 264: G1009–G1015, 1993
Wallis TS, Starkey WG, Stephen J, Haddon SJ, Osborne MP, Candy DCA: Enterotoxin production by Salmonella typhimurium strains of different virulence. J Med Microbiol 21: 19–23, 1986
Houston CW, Davis CP, Peterson JW: Salmonella toxin synthesis is unrelated to the presence of temperate bacteriophages. Infect Immun 35: 749–751, 1982
De SN, Chatterji DN: An experimental study on the mechanism of action of V. cholerae on the intestinal mucous membrane. J Pathol Bacteriol 66: 559–562, 1953
Evans DG, Evans DJ, Gorbach SL: Identification of enterotoxigenic E. coli and serum antitoxin activity by the vascular permeability factor assay. Infect Immun 8: 731–735, 1973
Peterson JW, Houston CW, Koo FCW: Influence of culture conditions on mitomycin C-mediated bacteriophage induction and release of Salmonella toxin. Infect Immun 32: 232–242, 1981
Kaur R, Ganguly NK, Kumar L, Walia BNS: Studies on the pathophysiological mechanism of Campylobacter jejuni induced fluid secretion in rat ileum. FEMS Microbiol Lett 111: 327–330, 1993
Kessler M, Acuto O, Sterelli C, Murer H, Mueller M, Semenza G: A modification procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta 506: 136–154, 1978
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1953
Pinkus LM: Separation and use of enterocytes. Meth Enzymol 77: 154–162, 1981
Toyoda S, Lee PC, Labenthol E: Physiological factors controlling release of enterokinase from rat enterocytes. Dig Dis Sci 30: 1174–1180, 1985
Ohkawa H, Oshishi N, Yagi H: Reaction of linoleic and hydroperoxide with TBA. J Lipid Res 19: 1053–1057, 1979
Recknagel RO, Glende EA: Spectrophometric determination of lipid conjugated dienes. Meth Enzymol 105: 331–337, 1984
Moron MS, Depierre JW, Mannerick B: Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat liver and lung. Biochim Biophys Acta 582: 67–78, 1979
Thurnham DI, Smith E, Flora SP: Concurrent liquid chromatographic assay of retinol, α-tocopherol, β-carotene, α-carotene, lycopene and β-cryptoxanthin in plasma with tocopherol acetate as internal standard. Clin Chem 34 (Part 1): 377–381, 1988
Hopfer U: Isolated membrane vesicles as tools for analysis of epithelial transport. Am J Physiol 233: E445–E449, 1977
Koo FCW, Peterson JW, Houston CW, Molina N: Pathogenesis of experimental salmonellosis. Inhibition of protein synthesis by cytotoxin. Infec Immun 43: 93–100, 1984
Phull PS, Green CJ, Jacyna MR: A radical view of the stomach, the role of oxygen derived free radicals and antioxidants in gastroduodenal disease. Eur J Gastroenterol Hepatol 327: 265–274, 1995
Parks DA: Oxygen radicals: mediators of gastrointestinal pathophysiology. Gut 30: 293–298, 1989
Otamiri T: Oxygen radicals, lipid peroxidation, and neutrophil infiltration after small intestinal ischemia and reperfusion. Surgery 105: 593–597, 1989
Kelly F: Glutathione content of the small intestine: regulation and function. Br J Nutr 69: 589–596, 1993
Jankowski J, Bridges AB, Scott N et al.: Circulating free radical markers and peptic ulcer disease. Eur J Gastroenterol Hepatol 3: 823–828, 1991
Hamilton R, Macleod J, Butler D: Functional and structural response of the intestine to enteric infections. In: S Tzipori (ed). Infectious Diarrhoea in the Young. Elsevier Science Publishers, B.V. (Biomedical Division), 1985, pp 165–171
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mehta, A., Singh, S., Dhawan, V. et al. Intestinal mucosal lipid peroxidation and absorptive function In Salmonella typhimurium mediated intestinal infection. Mol Cell Biochem 178, 345–352 (1998). https://doi.org/10.1023/A:1006891019115
Issue Date:
DOI: https://doi.org/10.1023/A:1006891019115