Abstract
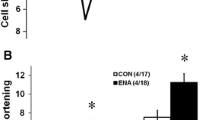
The aim of this study was to explore the possible participation of cardiac renin-angiotensin system (RAS) in the ischemia-reperfusion induced changes in heart function as well as Ca2+-handling activities and gene expression of cardiac sarcoplasmic reticulum (SR) proteins. The isolated rat hearts, treated for 10 min without and with 30 μM captopril or 100 μM losartan, were subjected to 30 min ischemia followed by reperfusion for 60 min and processed for the measurement of SR function and gene expression. Attenuated recovery of the left ventricular developed pressure (LVDP) upon reperfusion of the ischemic heart was accompanied by a marked reduction in SR Ca2+-pump ATPase, Ca2+-uptake and Ca2+-release activities. Northern blot analysis revealed that mRNA levels for SR Ca2+-handling proteins such as Ca2+-pump ATPase (SERCA2a), ryanodine receptor, calsequestrin and phospholamban were decreased in the ischemia-reperfused heart as compared with the non-ischemic control. Treatment with captopril improved the recovery of LVDP as well as SR Ca2+-pump ATPase and Ca2+-uptake activities in the postischemic hearts but had no effect on changes in Ca2+-release activity due to ischemic-reperfusion. Losartan neither affected the changes in contractile function nor modified alterations in SR Ca2+-handling activities. The ischemia-reperfusion induced decrease in mRNA levels for SR Ca2+-handling proteins were not affected by treatment with captopril or losartan. The results suggest that the improvement of cardiac function in the ischemic-reperfused heart by captopril is associated with the preservation of SR Ca2+-pump activities; however, it is unlikely that this action of captopril is mediated through the modification of cardiac RAS. Furthermore, cardiac RAS does not appear to contribute towards the ischemia-reperfusion induced changes in gene expression for SR Ca2+-handling proteins.
Similar content being viewed by others
References
Dhalla NS, Panagia V, Singal PK, Makino N, Dixon IMC, Eyolfson DA: Alterations in heart membrane calcium transport during the development of ischemia-reperfusion injury. J Mol Cell Cardiol 20(suppl III): 3–13, 1988
Morgan JP: Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med 325: 625–632, 1991
Krause S, Hess ML: Characterization of cardiac sarcoplasmic reticulum dysfunction during short-term, normothermic, global ischemia. Circ Res 55: 176–184, 1984
Krause SM, Jacobus WE, Becker LC: Alterations in cardiac sarcoplasmic reticulum calcium transport in the postischemic "stunned’ myocardium. Circ Res 65: 526–530, 1989
Wu QY, Feher JJ: Effect of ischemia and ischemia-reperfusion on ryanodine binding and Ca2+ uptake of cardiac sarcoplasmic reticulum. J Mol Cell Cardiol 27: 1965–1975, 1995
Wu QY, Feher JJ: Effect of ischemia on the function of ryanodine-sensitive cardiac sarcoplasmic reticulum. J Mol Cell Cardiol 29: 1363–1373, 1997
Zucchi R, Ronca-Testoni S, Yu G, Galbani P, Ronca G, Mariani M: Effect of ischemia and reperfusion on cardiac ryanodine receptorssarcoplasmic reticulum Ca2+ channels. Circ Res 74: 271–280, 1994
Dzau VJ: Cardiac renin-angiotensin system. Molecular and functional aspects. Am J Med 84: 22–27, 1988
Lindpaintner K, Jin M, Niedermaier N, Wilhelm MJ, Ganten D: Cardiac angiotensinogen and its local activation in the isolated perfused beating heart. Circ Res 67: 564–573, 1990
Noda K, Sasaguri M, Ideishi M, Ikeda M, Arakawa K: Role of locally formed angiotensin II and bradykinin in the reduction of myocardial infarct size in dogs. Cardiovasc Res 27: 334–340, 1993
Rogers TB, Lokuta AJ: Angiotensin II signal transduction pathways in the cardiovascular system. Trends Cardiovasc Med 4: 110–116, 1994
Dostal DE, Hunt RA, Kule CE, Bhat GJ, Karoor V, McWhinney CD, Baker KM: Molecular mechanisms of angiotensin II in modulating cardiac function: Intracardiac effects and signal transduction pathways. J Mol Cell Cardiol 29: 2893–2902, 1997
Sadoshima J, Izumo S: Signal transduction pathways of angiotensin II-induced c-fos gene expression in cardiomyocytes in vitro: Roles of phospholipid-derived second messengers. Circ Res 73: 424–438, 1993
Kim S, Ohta K, Hamagauchi A, Omura T, Yukimura T, Miura K, Inada Y, Ishimura Y, Chatani F, Iwao H: Angiotensin II type 1 receptor antagonist inhibits the gene expression of transforming growth factor beta-1 and extracellular matrix in cardiac and vascular tissues of hypertensive rats. J Pharmacol Exp Ther 273: 509–515, 1995
Yang BC, Phillips MI, Ambuehl PEJ, Shen LP, Mehta P, Mehta JL: Increase in angiotensin II type 1 receptor expression immediately after ischemia-reperfusion in isolated rat hearts. Circulation 96: 922–926, 1997
Yoshiyama M, Kim S, Yamagishi H, Omura T, Tani T, Yanagi S, Tada I, Teragaki M, Akioka K, Takeuchi K, Takeda T: Cardioprotective effect of the angiotensin II type 1 receptor antagonist TCV-116 on ischemia-reperfusion injury. Am Heart J 128: 1–6, 1994
Ju H, Scammell-LaFleur T, Dixon IMC: Altered mRNA abundance of calcium transport genes in cardiac myocytes induced by angiotensin II. J Mol Cell Cardiol 28: 1119–1128, 1996
Ertl G, Kloner RA, Alexander W, Braunwald E: Limitation of experimental infarct size by an angiotensin converting enzyme inhibitor. Circulation 65: 40–48, 1982
Li K, Chen X: Protective effects of captopril and enalapril on myocardial ischemia and reperfusion damage of rat. J Mol Cell Cardiol 19: 909–915, 1987
Fleetwood G, Boutinet S, Meier M, Wood JM: Involvement of the renin-angiotensin system in ischemic damage and reperfusion arrhythmias in the isolated perfused rat heart. J Cardiovasc Pharmacol 17: 351–356, 1991
Grover GJ, Sleph PG, Dzwonczyk R, Wang P, Fung W, Tobias D, Cushman DW: Effects of different angiotensin-converting enzyme (ACE) inhibitors on ischemic isolated rat hearts: relationship between cardiac ACE inhibition and cardioprotection. J Pharmacol Exp Ther 257: 919–929, 1991
Werrmann JG, Cohen SM: Comparison of effects of angiotensin-converting enzyme inhibition with those of angiotensin II receptor antagonism on functional and metabolic recovery in postischemic working rat heart as studied by (31P) nuclear magnetic resonance. J Cardiovasc Pharmacol 24: 573–586, 1994
Hunyady L, Balla T, Catt K: The ligand binding site of the angiotensin AT1 receptor. Trends Pharmacol Sci 17: 135–140, 1996
Persad S, Takeda S, Panagia V, Dhalla NS: β-adrenoceptor-linked signal transduction in ischemic-reperfused heart and scavenging of oxyradicals. J Mol Cell Cardiol 29: 545–558, 1997
Afzal N, Dhalla NS: Differential changes in left and right ventricular SR calcium transport in congestive heart failure. Am J Physiol 262: H868–H874, 1992
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951
Kaneko M, Beamish RE, Dhalla NS: Depression of heart sarcolemmal Ca2+-pump activity by oxygen free radicals. Am J Physiol 256: H368–H374, 1989
Tausky H, Shorr H. A microcoloric method for the estimation of inorganic phosphorus. J Biol Chem 202: 678–685, 1953
Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS: Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244: E528–E535, 1983
Chomczynski P, Sacchi N: Single step method of RNA isolation by acid guanine thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987
Davis MD, Lebolt W, Feher JJ: Reversibility of the effects of normothermic global ischemia on the ryanodine-sensitive and ryanodineinsensitive calcium uptake of cardiac sarcoplasmic reticulum. Circ Res 70: 163–171, 1992
Lindner M, Erdmann E, Beuckelmann DJ: Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol 30: 743–749, 1998
De Graeff PA, Van Gilst WH, De Langen CDJ, Kingma JH, Wesseling H: Concentration-dependent protection by captopril against ischemiareperfusion injury in the isolated rat heart. Arch Int Pharmacodyn Ther 280: 181–193, 1986
Rahusen FD, Van Gilst WH, Robillard GT, Dijkstra K, Wildevuur CRH: Captopril improves recovery of adenosine triphosphate during reperfusion of the ischemic isolated rat heart: A 31phosphorus-nuclear-magnetic-resonance study. Basic Res Cardiol 83: 540–549, 1988
Przyklenk K, Kloner RA: Relationships between structure and effects of ACE inhibitors: Comparative effects in myocardial ischaemic/reperfusion injury. Br J Clin Pharmacol 28: 167S–175S, 1989
Bagchi D, Iyengar J, Stockwell P, Das DK: Enhanced prostaglandin production in the ischemic-reperfused myocardium by captopril linked with its free radical scavenging action. Prostagland Leukotr Essent Fatty Acids 38: 145–150, 1989
Westlin W, Mullane K: Does captopril attenuate reperfusion induced myocardial dysfunction by scavenging free radicals? Circulation 77(suppl I): I30–I39, 1988
Liu X, Engelmann RM, Rousou JA, Cordis GA, Das DK: Attenuation of myocardial reperfusion injury by sulfhydryl-containing angiotensin converting enzyme inhibitors. Cardiovasc Drugs Ther 6: 437–443, 1992
Zweier JL, Flaherty JT, Weisfeldt ML: Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA 84: 1404–1407, 1987
Kukreja RC, Hess ML: The oxygen radical system: From equation through membrane protein interactions to cardiovascular injury and protection. Cardiovasc Res 26: 641–655, 1992
Schomig A, Dart AM, Dietz R, Mayer E, Kubler W: Release of endogenous catecholamines in the ischemic myocardium of the rat, part A, locally mediated release. Circ Res 55: 689–701, 1984
Abrahamsson T, Almgren O, Carlsson L. Ischemia-induced local release of myocardial noradrenaline. J Cardiovasc Pharmacol 7(suppl 5): 19–22, 1985
Xu KY, Zweier JL, Becker LC: Hydroxyl radical inhibits sarcoplasmic reticulum Ca2+ ATPase function by direct attack on the ATP binding site. Circ Res 80: 76–81, 1997
Okabe E, Hess ML, Oyama M, Ito H: Characterization of free radicalmediated damage of canine cardiac sarcoplasmic reticulum. Arch Biochem Biophys 225: 164–177, 1983
Rona G: Catecholamine cardiotoxicity. J Mol Cell Cardiol 17: 291–306, 1985
Tanonaka K, Kamiyama T, Takezono A, Sakai K, Takeo S. Beneficial effects of angiotensin II converting enzyme inhibitor on post-ischemic contractile function of perfused rat heart. J Mol Cell Cardiol 28: 1659–1670, 1996
Ford WR, Clanachan AS, Lopaschuk GD, Schulz R, Jugdutt BI: Intrinsic Ang II type I receptor stimulation contributes to recovery of postischemic mechanical function. Am J Physiol 274: H1524–H1531, 1998
Sun Y, Weber KT: Angiotensin II receptor binding following myocardial infarction in the rat. Cardiovasc Res 28: 1623–1628, 1994
Yosojima K, Kilgore KS, Washington RA, Lucchesi BR, McGeer PL: Complement gene expression by rabbit heart: Upregulation by ischemia and reperfusion. Circ Res 82: 1224–1230, 1998
Arai M, Tomaru K, Takizawa T, Sekiguchi K, Yokoyama T, Suzuki T, Nagai R. Sarcoplasmic reticulum genes are selectively down-regulated in cardiomyopathy produced by doxorubicin in rabbits. J Mol Cell Cardiol 30: 243–254, 1998
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takeo, S., Nasa, Y., Tanonaka, K. et al. Role of cardiac renin-angiotensin system in sarcoplasmic reticulum function and gene expression in the ischemic-reperfused heart. Mol Cell Biochem 212, 227–235 (2000). https://doi.org/10.1023/A:1007174803993
Issue Date:
DOI: https://doi.org/10.1023/A:1007174803993