Abstract
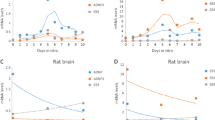
The applicability of antisense technology to suppress the expression of myelin associated glycoprotein (MAG) in cultured oligodendrocytes was evaluated. Differentiating oligodendrocyte precursor cells obtained by the shake-off method were exposed to nine unmodified antisense oligodeoxynucleotides (ODNs) targeted to the first seven exons of MAG mRNA. After four days, steady-state levels of MAG, proteolipid protein (PLP) and basic protein (BP) mRNAs were determined by Northern blot analysis. Only ODN annealing to 599-618 nt of the MAG mRNA (the junction of exon 5 and 6) resulted in a significant, 75% decrease in the MAG mRNA level. Unexpectedly, six other anti-MAG ODNs which had no significant effect on the MAG message, greatly increased the level of BP mRNA. The highest upregulation of approximately 12 fold was observed with ODN annealing to 139-168 nt (junction of exon 3 and 4). On the other hand, the 997-1016 ODN decreased the levels of BP and PLP messages by 70-80%. The 599-618 ODN also decreased the PLP mRNA by 85%. The results demonstrate that antisense ODNs targeted to one gene may profoundly alter the expression of other genes, and hence, complicate functional analysis of the targeted protein.
Similar content being viewed by others
REFERENCES
Chan Y.L., Olvera J., Wool I.G. (1983). The structure of a rat 28S ribosomal ribonucleic acid inferred from the sequence of nucleotides in a gene. Nucleic. Acid Res. 11:7819–7831.
Chiang M.Y., Chan H., Zounes M.A., Freier S.M., Lima W.F., Bennett, F. (1991). Antisense oligonucleotides inhibit intercellular adhesion molecule 1 expression by two distinct mechanisms. J. Biol. Chem. 266:18162–8171.
Chomczynski, P., Sacchi, N. (1987). Single-step method of RNA isolation by acidic guanidinium thiocyanatephenol-chloroform extraction. Anal. Biochem. 162:156–159.
Crooke S.T., Bennett, C.F. (1996). Progress in antisense oligonucleotide therapeutics. Annu. Rev. Pharmacol Toxicol. 36:107–129.
DeBellard, M.E., Tang, S., Mukhopadhyay, G., Shen, Y.J., Filbin, M.T. (1996). Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol. CellNeurosci. 7:89–101.
Grubinska, B., Laszkiewicz, I., Royland, J.E., Wiggins, R.C., Konat, G.W. (1994). Differentiation-specific demethylation of myelin associated glycoprotein gene in cultured oligodendrocytes. J. Neurosci Res. 39:233–242.
Gu, J., Royland, J.E., Wiggins, R.C., Konat, G.W. (1997). Selenium is required for normal upregulation of myelin genes in differentiating oligodendrocytes. J. Neurosci. Res. 47:626–635.
Han, J., Zhu, Z., Hsu, C., Finley, W.H. (1994). Selection of antisense oligonucleotides on the basis of genomic frequency of the target sequence. Antisense Res. Develop. 4:53–65.
Konat, G., Laszkiewicz, I., Bednarczuk, T.A., Kanoh, M., Wiggins, R.C. (1991). Generation of radioactive and nonradioactive ssDNA hybridization probes by polymerase chain reaction. Technique 3:311–319.
Lai, C., Brow, M.A., Nave, K.A., Noronha, A.B., Quarles, R.H., Bloom, F.E., Milner, R.J., Sutcliffe, J.G. (1987). Two forms of 1B236/myelin-associated glycoprotein, a cell adhesion molecule for postnatal neural development, are produced by alternative splicing. Pro. Natl. Acad. Sci., USA 84:4227–4241.
Laszkiewicz, I., Grubinska, B., Wiggins, R.C., Konat, G.W. (1997). Structural characterization of myelin associated glycoprotein gene core promoter. J. Neurosci. Res. 50:928–936.
McCarthy, K.D., de Vellis, J. (1980). Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell. Biol. 85:890–902.
McKerracker, L., David, S., Jackson, D.L., Kottis, V., Dunn, A.R., Braun, P.E. (1994). Identification of myelinassociated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 13:805–811.
Milligan, J.F., Matteucci, M.D., Martin, J.C. (1993). Current concepts in antisense drug design. J. Med. Chem. 36:1923–1937.
Milner, R.J., Lai, C., Nave, K.A., Lenoir, D., Ogata, J., Sutcliffe, J.G. (1985). Nucleotide sequences of two mRNAs for rat brain myelin proteolipid protein. Cell 42:931–939.
Mukhopadhyay, G., Doherty, P., Walsh, F.S., Crocker, P.R., Filbin, M.T. (1994). A novel role for myelinassociated glycoprotein as an inhibitor of axonal regeneration. Neuron. 13:757–767.
Newman, S., Kitamura, K., Campagnoni, A.T. (1987). Identification of a cDNA coding for a fifth form of myelin basic protein in mouse. Proc. Natl. Acad. Sci., USA 84:886–890.
Rathbone, M.P., Deforge, S., Deluca, B., Gabel, B., Laurenssen, C., Middlemiss, P., Parkinson, S. (1992a). Purinergic stimulation of cell division and differentiation: mechanisms and pharmacological implications. Med. Hypotheses 37:213–219.
Rathbone, M.P., Middlemiss, P.J., Gysbers, J.W., DeForge, S., Costello, P., Del Maestro, R. (1992b). Purine nucleosides and nucleotides stimulate proliferation of a wide range of cell types. In Vitro Cell Dev. Biol. 28A: 529–536.
Schafer, M., Fruttiger, M., Montag, D., Schachner, M., Martini, R. (1996). Disruption of the gene for the myelinassociated glycoprotein improves axonal regrowth along myelin in C57BL/Wld(s) mice. Neuron 16:1107–1113.
Sutcliffe, J.G., Milner, R.J., Shinnick, T.M., Bloom, F.E. (1983). Identifying the protein product of brain specific genes with antibodies to chemically synthesized peptides. Cell 33:671–682.
Weidner, D.A., Busch, H. (1994). Antisense phosphorothioate oligonucleotides direct both site-specific and nonspecific RNAse H cleavage of in vitro synthesized p120 mRNA. Oncol. Res. 6:237–242.
Woolf, T.M., Melton, D.A., Jannings, C.G. (1992). Specificity of antisense oligonucleotides in vivo. Proc. Natl. Acad. Sci., USA 89:7305–7309.
Ye, P., Kanoh, M., Zhu, W., Laszkiewicz, I., Royland, J.E., Wiggins, R.C., Konat, G. (1992). Cyclic AMP-induced upregulation of proteolipid protein and myelin associated glycoprotein gene expression in rat glioma C6 cells. J. Neurosci. Res. 31:578–583.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laszkiewicz, I., Wiggins, R.C. & Konat, G.W. Antisense Oligodeoxynucleotides Targeted to MAG mRNA Profoundly Alter BP and PLP mRNA Expression in Differentiating Oligodendrocytes: A Caution. Metab Brain Dis 14, 197–203 (1999). https://doi.org/10.1023/A:1020666826384
Issue Date:
DOI: https://doi.org/10.1023/A:1020666826384