Abstract
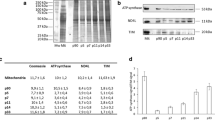
It has been suggested that phosphorylation of myelin basic protein (MBP) in CNS is catalyzed by protein kinase C (PKC). In order to demonstrate that PKC in the myelin phosphorylates MBP, PKC was partially purified from rat CNS myelin by solubilization with Triton X-100 followed by a DEAE-cellulose column. MBP and histone III-S were phosphorylated in the presence of Ca2+ and phospholipid by rat myelin PKC. High voltage electrophoresis revealed that the phosphoamino acids in MBP by this kinase was serine residue, which is known to be the amino acid phosphorylated by PKC. The activity of PKC extracted from myelin was inhibited by the addition of psychosine to the incubation mixture. To confirm the presence of PKC molecule and to identify the isoform of PKC in the myelin, the solubilized myelin fraction was applied on SDS-PAGE, transferred to a nitrocellulose sheet and stained with anti-PKC monoclonal antibodies. Rat CNS myelin contained the PKC of about 80 kDa (intact PKC), and no proteolytic fragments were observed. PKC isozymes in myelin were type II and III. A developmental study from 14 to 42 postnatal days showed that PKC activity in CNS myelin seemed to parallel the deposition of myelin protein.
Similar content being viewed by others
References
Carnegie, P.R., Kemp, P.E., Dunkley, P.R., and Murray, A.W. 1973. Phosphorylation of myelin basic protein by an adenosine 3′∶5′-cyclic monophosphate dependent protein kinase. Biochem. J. 135:569–572.
Carnegie, P.R., Dunkley, P.R., Kemp, P.E., and Murray, A.W. 1974. Phosphorylation of selected serine and threonine residues in myelin basic protein by endogenous and exogenous protein kinases. Nature 249:147–150.
Miyamoto, E., and Kakiuchi, S. 1974. In vitro and in vivo phosphorylation of myelin basic protein by endogenous and exogenous adenosine 3′∶5′-monophosphate dependent protein kinase in brain. J. Biol. Chem. 249:2769–2777.
Steck, A.J., and Appel, S.H. 1974. Phosphorylation of myelin basic protein. J. Biol. Chem. 249:5416–5420.
Agrawal, H.C., Randle, C.L., and Agrawal, D. 1981. In vivo phosphorylation of two myelin basic proteins of developing rabbit brain. J. Biol. Chem. 256:12243–12246.
Wise, B.C., Glass, D.B., Chou, C.-H.J., Raynor, R.L., Katoh, N., Schatzman, R.C., Turner, R.S., Kibler, R.F., and Kuo, J.F. 1982. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II. Substrate specificity and inhibition by various agents. J. Biol. Chem. 257:8489–8495.
Murray, N., and Steck, A.J. 1984. Impulse conduction regulates myelin basic protein phosphorylation in rat optic nerve. J. Neurochem. 43:243–248.
Stoner, G.L. 1984. Predicted folding of β-structure in myelin basic protein. J. Neurochem. 43:433–447.
Vartanian, T., Szuchet, S., Dawson, G., and Campagnoni, A.T. 1986. Oligodendrocyte adhesion activates protein kinase C-mediated phosphorylation of myelin basic protein. Science 234:1395–1398.
DesJardins, K.C., and Morell, P. 1983. Phosphate groups modifying myelin basic proteins are metabolically labile; methyl groups are stable. J. Cell Biol. 97:438–446.
Turner, R.S., Chou, C.-H.J., Kibler, R.F., and Kuo, J.F. 1982. Basic protein in brain myelin is phosphorylated by endogenous phospholipid-sensitive Ca2+-dependent protein kinase. J. Neurochem. 39:1397–1404.
Wu, N.-C., and Ahmad, F. 1984. Calcium- and cyclic AMP-regulated protein kinases of bovine central-nervous-system myelin. Biochem. J. 218:923–932.
Ulmer, J.B., and Braun, P.E. 1987. Chloroform markedly stimulates the phosphorylation of myelin basic proteins. Biochem. Biophys. Res. Commun. 146:1084–1088.
Yoshimura, T., Kobayashi, T., Shinnoh, N., and Goto, I. 1990. Accumulation of galactosylsphingosine (psychosine) does not interfere with phosphorylation and methylation of myelin basic protein in the twitcher mouse. Neurochem. Res. 15:963–967.
Hannun, Y.A., and Bell, R.M. 1987. Lysosphingolipids inhibits protein kinase C: implications for the sphingolipidoses. Science 235:670–674.
Girard, P.R., Mazzei, G.L., Wood, J.G., and Kuo, J.F. 1985. Polyclonal antibodies to phospholipid/Ca2+-dependent protein kinase and immunocytochemical localization of the enzyme in rat brain. Proc. Natl. Acad. Sci. USA 82:3030–3034.
Hidaka, H., Tanaka, T., Onoda, K., Hagiwara, M., Watanabe, M., Ohta, H., Ito, Y., Tsurudome, M., and Yoshida, T. 1988. Cell type-specific expression of protein kinase C isozymes in the rabbit cerebellum. J. Biol. Chem. 263:4523–4526.
Agrawal, H.C., Burton, R.M., Fishman, M.A., Mitchell, R.F., and Prensky, A.L. 1972. Partial characterization of a new myelin component. J. Neurochem. 19:2083–2089.
Yoshimura, T., Kobayashi, T., Mitsuo, K., and Goto, I. 1989. Decreased fatty acylation of myelin proteolipid protein in the twitcher mouse. J. Neurochem. 52:836–841.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
Towbin, H., Staehelin, T., and Gordon, J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350–4354.
Allerton, S.E., and Perlmann, G.E. 1965. Chemical characterization of the phosphoprotein Phosvitin. J. Biol. Chem. 240:3892–3898.
Wroblewski, F., and LaDue, J.S. 1955. Lactate dehydrogenase activity in blood. Proc. Soc. Exp. Biol. & Med. 90:210–213.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. 1951. Protein measurement with Folin phenol reagents. J. Biol. Chem. 193:265–275.
Murray, N., and Steck, A.J. 1986. Activation of myelin protein kinase by diacylglycerol and 4β-phorbol 12-myristate 13-acetate. J. Neurochem. 46:1655–1657.
Turner, R.S., Chou, C.-H.J., Mazzei, G.J., Dembure, P., and Kuo, J.F. 1984. Phospholipid-sensitive Ca2+-dependent protein kinase preferentially phosphorylates serine-115 of bovine myelin basic protein. J. Neurochem. 43:1257–1264.
Kishimoto, A., Nishiyama, K., Nakanishi, H., Uratsuji, Y., Nomura, H., Takeyama, Y., and Nishizuka, Y. 1985. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3′∶5′-monophosphate-dependent protein kinase. J. Biol. Chem. 260:12492–12499.
Kishimoto, A., Kajiwara, N., Shiota, M., and Nishizuka, Y. 1983. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J. Biol. Chem. 258:1156–1164.
Melloni, E., Pontremoli, S., Michetti, M., Sacco, O., Sparatore, B., Salamine, F., and Horecker, B.L. 1985. Binding of protein kinase C to neutrophil membranes in the presence of Ca2+ and its activation by a Ca2+-requiring proteinase. Proc. Natl. Acad. Sci. USA 82:6435–6439.
Smith, M.E., and Benjamins, J.A. 1984. Model systems for study of perturbations of myelin metabolism, in Myelin (Morell, P., ed), pp 245–258, Plenum Press, New York.
Kikkawa, U., Ogita, K., Ono, Y., Asaoka, Y., Shearman, M.S., Fujii, T., Sekiguchi, K., Igarashi, K., and Nishizuka, Y. 1987. The common structure and activities of four subspecies of rat brain protein kinase C. FEBS Lett. 223:212–216.
Stichel, C.C., and Singer, W. 1988. Localization of isoenzymes II/III of protein kinase C in rat brain visual cortex (area 17), hippocampus and dentate gyrus. Exp. Brain Res. 72:443–449.
Nishizuka, Y. 1989. The family of protein kinase C for signal transduction. JAMA 262:1826–1833.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoshimura, T., Kobayashi, T. & Goto, I. Protein kinase C in rat brain myelin. Neurochem Res 17, 1021–1027 (1992). https://doi.org/10.1007/BF00966831
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00966831