Abstract
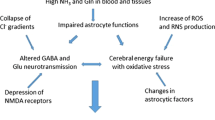
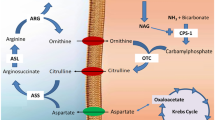
The effect of a recent hyperammonemic model, consisting of a high ammonia diet for 3, 7, 15, 45, and 90 days, on glial fibrillary acidic protein (GFAP) in the rat spinal cord and on blood ammonia levels has been studied. The high ammonia diet was prepared by mixing a standard diet with ammonium acetate (20% wt/wt); in addition, 5 mM of ammonium acetate was added to the water supply. GFAP contents were determined by means of immunoblotting analysis. The results demonstrated that this high ammonia diet model neither induces significant changes in GFAP immunoreactivity, nor modifies total protein concentration, and only induces significant blood hyperammonemic levels in the first days of treatment. An adaptative response to the diet is suggested and discussed to explain these results. A relation between ammonia and GFAP expression is suggested because transient hyperammonemia induces transient, although no significant, changes on GFAP expression.
Similar content being viewed by others
References
Cooper, A. J. L., and Plum, F. 1987. Biochemistry and physiology of brain ammonia. Physiol. Rev. 67:440–519.
Schenker, S. 1989. Hepatic encephalopathy: the present and future. Pages, 3–24,in Butterworth, R. F., and Pomier, G. (eds.), Hepatic encephalopathy. Pathophysiology and treatment, Humana, Clifton, NJ.
Basile, A. S., Jones, E. A., and Skolnick, P. 1991. The pathogenesis and treatment of hepatic encephalopathy: evidence for the involvement of benzodiazepine receptor ligands. Pharmacol. Rev. 43:27–71.
Butterworth, R. F., Giguère, J. F., Michaud, J., Lavoie, J., and Pomier, G. 1987. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem. Pathol. 6:1–12.
Jessy, J., Mans, A. M., DeJoseph, M. R., and Hawkins, R. A. 1990. Hyperammonaemia causes many of the changes found after portacaval shunting. Biochem. J. 272:311–317.
Batshaw, M. L., and Monahan, P. S. 1987. Treatment of urea cycle disorders. Enzyme 38:242–250.
Diemer, N. H., and Laursen, H. 1977. Glial cell reactions in rats with hyperammonemia induced by urease or porto-caval anastomosis. Acta Anat. Scand. 55:425–442.
Batshaw, M. L., Hyman, S. L., Mellits, E. D., Thomas, G. H., DeMuro, R., and Coyle, J. T. 1986. Behavioural and neurotransmitter changes in urease-infused rats: a model of congenital hyperammonemia. Pediatr. Res. 20:1310–1315.
Gutiérrez, J. A., and Norenberg, M. D. 1975. Alzheimer II astrocytosis following methionine sulfoximine. Arch. Neurol. 32:123–126.
Yamamoto, T., Iwasaki, Y., Sato, Y., Yamamoto, H., and Konno, H. 1989. Astrocyte pathology of methionine sulfoximine-induced encephalopathy. Acta Neuropathol. 77:357–368.
Hawkins, R. A., and Jessy, J. 1991. Hyperammonaemia does not impair brain function in the absence of net glutamine synthesis. Biochem. J. 277:697–703.
Takahashi, H., Koehler, R. C., Brusilow, S. W., and Traystman, R. J. 1991. Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats. Am. J. Physiol. 261:H825-H829.
Azorín, I., Miñana, M. D., Felipo, V., and Grisolía, S. 1989. A simple animal model of hyperammonemia. Hepatology 10:311–314.
Norenberg, M. D. 1987. The role of astrocytes in hepatic encephalopathy. Neurochem. Pathol. 6:13–33.
O'Callaghan, J. P. 1991. Assessment of neurotoxicity: use of glial fibrillary acidic protein as a biomarker. Biomed. Environ. Sci. 4:197–206.
O'Callaghan, J. P. 1992. Assessment of neurotoxicity using assays of neuron- and glia-localized proteins: chronology and critique. Pages 83–100,in Tilson, H., and Mitchell, C. (eds.), Neurotoxicology, Raven Press, New York.
Zamora, A. J., Cavanagh, J. B., and Kyu, M. H. 1973. Ultrastructural responses of the astrocytes to porto-caval anastomosis in the rat. J. Neurol. Sci. 18:25–45.
Norenberg, M. D., and Lapham, L. W. 1974. The astrocyte response in experimental portal-systemic encephalopathy: an electron microscopic study. J. Neuropathol. Exp. Neurol. 33:422–435.
Sobel, R. A., DeArmond, S. J., Forno, L. S., and Eng, L. F. 1981. Glial fibrillary acidic protein in hepatic encephalopathy. J. Neuropathol. Exp. Neurol. 40:625–632.
Kretzschmar, H. A., DeArmond, S. J., and Forno, L. S. 1985. Measurement of GFAP in hepatic encephalopathy by ELISA and transblots. J. Neuropathol. Exp. Neurol. 44:459–471.
Kimura, T., and Budka, H. 1986. Glial fibrillary acidic protein and S-100 protein in human hepatic encephalopathy: immunocytochemical demonstration of dissociation of two glia associated proteins. Acta Neuropathol. 70:17–21.
Bodega, G., Suárez, I., Rubio, M., Arilla, E., and Fernández, B. 1991. Heterogeneous astroglial response in the rat spinal cord to long-term portacaval shunt. An immunohistochemical study. Glia 4:400–407.
Suárez, I., Bodega, G., Arilla, E., Rubio, M., Villalba, R. M., and Fernández, B. 1992. Different response of astrocytes and Bergmann glial cells to portacaval shunt. An immunohistochemical study in the rat cerebellum. Glia 6:172–179.
Pearson, D. 1973. Laboratory techniques in food analysis. Butterworth and Co. Ltd., London.
Meikle, A., and Martin, A. H. 1981. A rapid method for removal of the spinal cord. Stain Technol. 56:235–237.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Kosenko, E., Felipo, V., Miñana, M. D., Grau, E., and Grisolía, S. 1991. Ammonium ingestion prevents depletion of hepatic energy metabolites induced by acute ammonium intoxications. Arch. Biochem. Biophys. 290:484–488.
Girard, G., and Butterworth, R. F. 1992. Effect of portacaval anastomosis on glutamine synthetase activities in liver, brain, and skeletal muscle. Dig. Dis. Sci. 37:1121–1126.
Bodega, G., Suárez, I., Rubio, M., Villalba, R. M., and Fernández, B. 1992. Hyperammonemia induces transient GFAP immunoreactivity changes in the goldfish spinal cord (Carassius auratus L.). Neurosci. Res. 13:217–225.
Wasterlain, C. G., Lockwood, A. H., and Conn, M. 1973. Chronic inhibition of brain protein synthesis after portocaval shunting. Neurology 28:233–238.
Schott, K., Poetter, V., and Neuhoff, V. 1984. Ammonia inhibits protein synthesis in slices from young rat brain. J. Neurochem. 42:644–646.
Felipo, V., Miñana, M. D., Azorín, I., and Grisolía, S. 1988. Induction of rat brain tubulin following ammonium ingestion. J. Neurochem. 51:1041–1045.
Norenberg, M. D., Neary, J. T., Norenberg, L. O., and McCarthy, M. 1990. Ammonia induced decrease in glial fibrillary acidic protein in cultured astrocytes. J. Neuropathol. Exp. Neurol. 33:422–435.
Miñana, M. D., Felipo, V., Quel, A., Pallardo, F., and Grisolía, S. 1989. Selective regional distribution of tubulin induced by hyperammonemia. Neurochem. Res. 14:1241–1243.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bodega, G., Suárez, I., Boyano, M.C. et al. High ammonia diet: Its effect on the glial fibrillary acidic protein (GFAP). Neurochem Res 18, 971–975 (1993). https://doi.org/10.1007/BF00966755
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00966755