Abstract
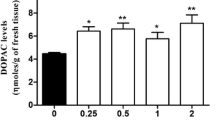
The effects of chronic dietary sodium chloride (NaCl) consumption on renal function and brain dopamine receptors were studied in adult, male normotensive rats. Compared to rats maintained on the normal NaCl (0.33%) diet, animals maintained on the low NaCl (0%) diet for 4 weeks exhibited significant increases in plasma aldosterone, chloride and changes in urinary electrolyte excretion. In contrast, rats maintained on the high NaCl (8%) diet for 4 weeks demonstrated significant increases in urine volume and urinary sodium, chloride and dopamine excretions and water intake. Rats fed the high NaCl diet displayed a 42–59% decrease (p<0.001–0.05) in D1 binding in the nucleus accumbens (NA), olfactory tubercle (OT) and the striatum (STM), without any effects on D2 binding in these brain regions. Rats maintained on the low NaCl diet also demonstrated decreased D1 binding in the ventral (24%, p<0.02) and lateral (29%, p<0.01) STM, but not in the OT, NA, entopeduncular nucleus and substantia nigra. Rats fed low or high NaCl diets exhibited a 35–180% increase (p<0.01–0.05) in D2 binding in several mid-brain areas (e.g. hypothalamus, thalamus and hippocampus) and hindbrain regions (e.g. superior colliculus and nucleus tractus solitarius) without affecting the D1 binding. These data indicate that chronic modification of dietary salt intake profoundly affects the renal handling of sodium/water excretion and leads to selective up- and/or down-regulation of DA receptor subtypes in different areas of the brain. These findings may have relevance to centrally-mediated hypertension, Parkinson's disease, schizophrenia and other brain disorders involving dopamine and dopamine receptors.
Similar content being viewed by others
References
Seeman, P. 1980. Brain dopamine receptors. Pharmacol. Rev. 32: 229–313.
Goldberg, L. I., and Kohli, J. D. 1983. Peripheral dopamine receptors: a classification based on potency series and specific antagonism. Trends Pharmacol. Sci. 4:64–68.
Sokoloff, P., Giros, B., Martres, M.-P., Bouthenet, M-L., and Schwartz, J-C. 1990. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347:146–151.
Von Tol, H. H. M., Bunzow, J. R., Guan, H-C., Niznik, H. B., and Civelli, O. 1991. Cloning of the gene for a human D4 receptor with high affinity for the antipsychotic clozapine. Nature 350:610–614.
Sunhara, R. K., Guan, H-C., O'dowd, B. F., Seeman, P., Laurier, L. G., Ng, G., George, S. R., Torchia, J., Van Tol, H. H. M., and Niznik, H. B. 1991. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350: 614–619.
Goldberg, L. I., The Pharmacological basis of clinical use of dopamine. Proc. Roy. Soc. Med. 70 (Suppl. 2): 7–15 1977.
Lokhandwala, M. F., and Jandhyala, B. S. 1979. The role of sympathetic nervous system in the vascular actions of dopamine. J. Pharmacol. Expt. Ther. 210:120–126.
Kohli, J. D., Mcnay, J. L., Rajfer, S. I., and Murphy, M. B. 1991. Peripheral dopamine receptors in cardiovascular therapy. The legacy of Leon Goldberg (1927–1989). Hypertension 17:700–706.
Morgnov, N., and Baines, A. D. 1985. Vagal afferent activity and renal nerve release of dopamine. Ca. J. Physiol. 63:636–641.
Huang, W-C., Tsai, L-M., and Wu, J-N. 1988. Effects of renal denervation on bilateral renal response to saline loading in anteroventral third ventricle-lesioned rats. Brain Res. 460:83–93.
Huang, B., Malvin, R. L., Lee, J., and Grekin, R. J. 1987. Central dopaminergic regulation of aldosterone secretion in sheep. Hypertension 10:157–163.
Lichardus, B., Oklolocany, J., Mckinley, M. J., Denton, D. A., and Ponec, J. 1987. Brain involvement in the regulation of renal sodium excretion. Klin. Wochenscher 65 (Suppl VIII): 33–39.
Hansell, P., Sjoquist, M., Fasching, A., Isakson, B., Karlsson, M. and Ulfendahl, H. R. 1988. CNS-induced natriuresis during dopamine receptor blockade. Further support for the existence of at least two separate natriuretic hormonal systems. Acta Physiol. Scand. 1:1–16.
Stumpe, K. O., Kolloc, R., Higuchi, M., Kruck, F., and Vetter, H. 1977. Hyperprolactinamia and antihypertensive effects of bromocriptine in essential hypertension: identification of abnormal central dopamine control. Lancet 2:211–214.
Fitzsimons, J. T., and Settle, P. E. 1975. The relative importance of central nervous catecholaminergic and cholinergic mechanisms in drinking in response to angiotensin and other thirst stimuli. J. Physiol. 250:613–631.
Yoshimura, M., Ikegaki, I., Nishimura, M., and Takhasi, H. 1991. Role of dopaminergic mechanisms in the kidney for the pathogenesis of hypertension. J. Auton. Pharmacol. 10 (suppl. 1):s67-s72.
Oates, N. S., Ball, S. G., Perkins, C. M., and Lee, M. R. 1979. Plasma and urine dopamine in man given sodium chloride in the diet. Clin. Sci. 56:261–264.
Hegde, S. S., Jadhav, A. L., and Lokhandwala, M. F. 1989. Role of kidney dopamine in the natriuretic response to volume expansion in rats. Hypertension 13:828–834.
Clark, B. A., Rosa, R. M., Epstein, F. H., Young, J. B. and Landberg, L. 1991. Altered dopaminergic responses in hypertension. Hypertension 19:589–594.
Rosenkranz, R. P., Sharif, N. A., Corkins, S. F., Lakatos, I., Lake, K. D., and Mcclelland, D. L. 1990. Modification of dietary sodium chloride intake: effects on diuresis and endogenous dopamine activity in the rat. Eur. J. Pharmacol. 183:1051p.
Sharif, N. A., Nunes, J. L., Rapp, J. M., Mcclelland, D. L., Rosenkranz, R. P., and Whiting, R. L. 1990. Quantitative autoradiography of rat brain and kidney dopamine D1 and D2 receptors: regulation by dietary salt. Dopamine'90, Satellite Meeting of XI IUPHAR, Como, Italy, Abst.#90.
Altar, A. C., and Marien, M. C. 1987. Picomolar affinity of [125I]SCH23982 for D1 receptors in brain demonstrated with digital subtraction autoradiography. J. Neurosci. 7:213–222.
Matres, M-P., Bouthenet, M-L., Sales, N., Sokoloff, P., and Schwartz, J-C. 1985. Widespread distribution of brain dopamine receptors evidenced with [125I]Iodosulpiride, a highly selective ligand. Science 228:752–755.
Sharif, N. A., and Eglen, R. M. 1993. Quantitative autoradiography: a tool to visualize and quantify receptors, enzymes, transporters, and second messenger systems. pages 71–138,in, Sharif, N. A. (ed.), Molecular Imaging in Neuroscience: a practical approach. Oxford University Press (IRL Press), Oxford, U.K.
Sharif, N. A., and Hughes, J. 1989. Discrete mapping of brain mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides 10:499–522.
McClanahan, M., Sowers, J. R., Beck, F. W. J., Mohanty, P. K., and Mckenzie, T. 1985. Dopaminergic regulation of natriuretic response to acute volume expansion in dogs. Clin. Sci. 68:263–269.
Saavedra, J. M., Castren, E., Gutkind, J. S., and Nazareli, A. J. 1991. Regulation of brain atrial natriuretic peptide and angiotensin receptors: quantitative autoradiographic studies. Int. Rev. Neurobiol. 31:257–296.
Cachofeiro, V., Schiffrin, E. L., Bonhomme, M-C., Cantin, M., and Garcia, R. 1990. In vivo heterologous regulation of rat glomerular and vascular atrial natriuretic factor receptors by angiotensin II. J. Hypertension 8:1077–1083.
Chen, C-J., Apparsundaram, S., and Lokhandwala, M. F. 1990. Intrarenally produced angiotensin II opposes the natriuretic action of the dopamine-1 receptor agonist fenoldopam. J. Pharmacol. Expt. Ther. 256:486–491.
Bealer, S. L., Haywood, J. R., Gruber, K. A., Buckalew, V. M. Jr., Fink, G. D., Brody, M. J., and Johnson, A. K. 1983. Preoptic-hypothalamic periventricular lesions reduce natriuresis to volume expansion. Am. J. Physiol. 244:R51-R57.
Nagahama, S., Ann, H. S., Chen, Y-F., Lindheimer, M. L., and Oparil, S. 1987. Role of vasopressin in the cardiovascular effects of LY171555, a selective dopamine D2 receptor agonist: studies in conscious Brattleboro and Long-Evans rats. J. Pharmacol. Expt. Ther. 242:143–151.
Sofroniew, M. V. 1980. Projection from vasopressin, oxytocin and neurophysin neurones to neural targets in rat and human. J. Histochem. Cytochem. 28:248–253.
Torres, M., Barbella, Y. and Israel, A. 1989. Dopaminergic mediation of the diuretic and natriuretic action of centrally administered rat atrial natriuretic factor (99–126). Proc. Soc. Exp. Biol. Med. 190:18–22.
Jandhyala, B. S., Lokhandwala, M. F., Kivlighn, S. D., Ansari, A. F., and De Feo, M. L. 1987. Intracisternal administration of pergolide, a dopamine receptor agonist, triggers the release of an inhibitor of ouabain-sensitive sodium, potassium-dependent adenosine triphosphatase and enhances vascular reactivity in anaesthetized dogs. Clin. Sci. 73:183–188.
Jadhav, A. L., Lokhandwala, M. F., Ricci, A., and Amenta, F. 1991. Alterations in kidney dopamine production and dopamine receptors during increased sodium intake. J. Auton. Pharmacol 10 (suppl.1):130p.
Wyss, J. M., and Donovan, M. K. 1984. A direct projection from the kidney to the brainstem. Brain Res. 298:130–134.
Rowe, J. W., Shelton, R. L., Heldman, J. H., Vestal, R. E., and Robertson, G. L. 1979. Influence of the emetic reflex on vasopressin release in man. Kidney Int. 16:729–735.
Allin, R., Mintz, M., Russell, V., Engelbrecht, A., Lamm, M., Daniels, W., van der Spuy, G., Jaffer, A., Kellaway, L., and Taljaard, J. 1994. Effect of amygdaloid kindling on rat striatal dopamine D1- and D2-receptors. Neurochem. Res. 19:827–831.
Sharif, N. A., Nunes, J. L., Lake, K. D., McClelland, D. L., Lakatos, I., Corkins, S. F., Rosenkranz, R. P., Whiting, R. L., and Eglen, R. M. 1995. Chronic manipulation of dietary salt modulates renal physiology and dopamine receptor subtypes: functional and quantitative autoradiographic studies. Gen Pharmacol. (in press).
Seeman, P., and Niznik, H. B. 1990. Dopamine receptors and transporters in Parkinson's disease and schizophrenia. FASEB J. 4:2737–2744.
Nunes, J. L., Sharif, N. A., Michel, A. D., and Whiting, R. L. 1991. Dopamine D2-receptors mediate hypothermia in mice: ICV and IP effects of agonists and antagonists. Neurochem. Res. 16: 1167–1174.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sharif, N.A., Nunes, J.L., Rosenkranz, R.P. et al. Quantitative autoradiography demonstrates selective modulation of rat brain regional dopamine (D1 and D2) receptor subtypes after chronic manipulation of dietary salt. Neurochem Res 20, 121–128 (1995). https://doi.org/10.1007/BF00970535
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00970535