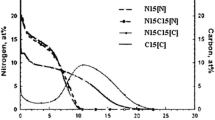
Abstract
Nine commercial high-temperature alloys were reacted with NH3 and with N2-5%H2 at temperatures of 1000–1200°C. In all cases extensive internal nitridation developed according to parabolic kinetics. Reaction with N2 produced precipitates of Cr2N plus, at 1000°C, some external CrN, in accordance with thermodynamic prediction. However NH3 produced an external scale of CrN and a near-surface zone of internal CrN as well as a deeper zone of Cr2N at 1100 and 1200°C. The CrN phase is metastable, and results from catalytic dissociation of NH3, which produces a high effective nitrogen activity. This high activity also leads to faster internal-precipitation reactions, whereas reaction rates in nitrogen are in reasonable agreement with Wagner's model of rate control by inwardly diffusing nitrogen at its equilibrium solubility. Precipitate morphologies are complex, reflecting the importance of the energy barrier to homogeneous nucleation.
Similar content being viewed by others
References
J. J. Barnes and G. Y. Lai, inCorrosion and Particle Erosion at High Temperatures, V. Srinivasan and K. Vedula (eds.) (TMS, Warrendale, PA, 1989), p. 617.
K. Tjokro, D. J. Young, R. Johansson, and J. D. Redmond,Corrosion 91, paper 548, (NACE, Houston, 1991).
D. L. Douglass,J. Met. 43, 74 (1991).
B. Mortimer, P. Grieveson, and K. H. Jack,Scan. J. Met.,1, 203 (1972).
D. C. Unthank, J. H. Driven, and K. H. Jack,Met. Sci. 8, 209 (1974).
G. Y. Lai,J. Met. 43, 54 (1991).
O. Kubaschewski and C. B. Alcock,Metallurgical Thermochemistry 5th ed. (Pergamon, 1983).
L. E. Kindlimann and G. S. Ansell,Met. Trans. 1, 163 (1970).
H. A. Wriedt and O. D. Gonzalez,Trans. AIME 221, 532 (1961).
H. J. Grabke and E. M. Petersen,Scripta Met. 12, 1111 (1978).
C. Wagner,Corros. Sci. 8, 889 (1968).
H. J. Goldschmidt,Insterstitial Alloys (Plenum, New York, 1967).
I. C. Chen and D. L. Douglass,Oxid. Met. 34, 473 (1990).
C. Wagner,Z. Elektrochem. 63, 772 (1959).
R. A. Rapp,Corrosion 21, 382 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tjokro, K., Young, D.J. Comparison of internal nitridation reactions in ammonia and in nitrogen. Oxid Met 44, 453–474 (1995). https://doi.org/10.1007/BF01058247
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01058247