Abstract
Purpose. The aim of this study was to enhance the transdermal absorption of the highly active progestin gestodene from matrix type transdermal delivery systems (TDDS) by formation of prodrugs with improved matrix solubility.
Methods. Gestodene esters were synthesized via acylation of the drug with the respective carboxylic anhydrides. Subsequently TDDS were produced using the solvent cast method. Selected formulations were examined with in vitro diffusion experiments using skin of nude mice.
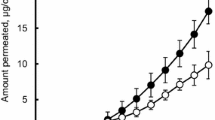
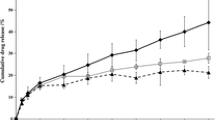
Results. One prodrug, gestodene caproate proved to be an oil at ambient temperature and showed a very high solubilty of over 10.5% in the TDDS matrix. Within in vitro penetration studies using those systems the prodrug exhibited a significantly higher transdermal penetration rate than gestodene from reference systems. Furthermore, the prodrug was hydrolyzed to the parent drug to a high extent during the passage of the skin.
Conclusions. Designing prodrugs to the requirements of matrix TDDS is an efficient way of enhancing the transdermal drug flux rate.
Similar content being viewed by others
REFERENCES
P. Mauvais-Jarvis. C. F. H. Vickers, and S. Wepierre. Percutaneous absorption of steroids, Academic Press, London, 1980.
H-D. Taubert and H. Kuhl. Das Klimakterium, Georg Thieme Verlag, Stuttgart, 1987.
D. R. Friend. Transdermal Delivery of Contraceptives. Crit. Rev. in Ther. Drug Carrier Syst. 7:149–186 (1990).
M. Elstein. Gestodene, The Parthenon Publishing Group, Carnforth, UK, 1987.
C. Günther. In vitro-und in vivo-Untersuchungen zur perkutanen Resorption von Estrogenen und Gestagenen als Grundlage für die Entwicklung eines transdermalen therapeutischen Systems. Ph. D. Thesis, Freie Universität Berlin, Berlin (1990).
C. Günther, U. Täuber, K. Schmidt-Gollwitzer, J. Riedl, and J. W. Tack. Transdermal steroid hormone compositions. DE 3,836,862, 1990, Schering AG: Chem. Abstr. 113:218251f (1990).
Y. W. Chien. Systemic delivery of pharmacologically active molecules across the skin. In R. L. Juliano (ed.) Targeted Drug Delivery, Springer-Verlag, Berlin, Heidelberg, New York, 1991, pp. 181–230.
J. Yu, T. Chien, and Y. W. Chien. Transdermal dual-controlled delivery of testosterone and estradiol: (1) impact of system design. Drug. Dev. Ind. Pharmacy 17:1883–1904 (1991).
R. Lipp and A. Müller-Fahrnow. X-ray structure determinations of crystals grown in transdermal delivery systems containing estradiol or gestodene. Pharm. Res. 11:S-213 (1994).
R. Lipp, J. Riedl, and J. W. Tack. Transdermal therapeutic systems containing crystallization inhibitors. WO 93/08795, 1993, Schering AG, Chem. Abstr. 119:P15357w (1993).
R. Lipp. Selection and use of crystallization inhibitors for steroid loaded transdermal delivery systems. Eur. J. Pharm. Biopharm. 40(suppl):85 (1994).
H. Hofmeister, R. Wiechert, K. Annen, H. Laurent, and H. Steinbeck. Δ15-17α-Äthinyl-steroide der Östranreihe, Verfahren zu ihrer Herstellung sowie diese enthaltende pharmazeutische Präparate, DE 2,546,062, 1977; Schering AG, Chem. Abstr. 87:168265 (1977).
H. Hofmeister, K. Annen, H. Laurent, K. Petzoldt, and R. Wiechert. Synthesen von Gestoden. Arzneim.-Forsch. (Drug Res.) 36:781–783 (1986).
R. Lipp, H. Laurent, C. Günther, J. Riedl, P. Esperling, and U. Täuber. Transdermal formulations containing gestodene esters. DE 4,329,242, 1995, Schering AG; Chem. Abstr. 122:248319 (1995).
B. W. Barry. Dermatological formulations, Marcel Dekker, New York, 1983.
K. B. Sloan (ed.). Prodrugs, topical and ocular drug delivery. Marcel Dekker, New York, 1992 (J. Swarbrick, Ed., Drugs and the pharmaceutical sciences; Vol. 53).
K. H. Valia, K. Tojo, and Y. W. Chien. Long-term permeation kinetics of estradiol: (III) kinetic analysis of the simultaneous skin permeation and bioconversion of estradiol esters. Drug Dev. Ind. Pharmacy 11:1133–1173 (1985).
K. Junkmann and H. Witzel. Chemie und Pharmakologie von Steroidhormon-Estern. Z. Vitam. Horm. Fermentforsch. 9:97–143 (1958).
D. Gould, L. Finckenor, E. B. Hershberg, J. Cassidy, and P. L. Perlman. Long-acting testosterone esters. Some considerations on their biological utilization. J. Am. Chem. Soc. 79:4472–4475 (1957).
R. J. Scheuplein and R. L. Bronaugh. In: L. A. Goldsmith (ed.) Biochemistry and Physiology of the Skin. Oxford University Press, New York, 1983, pp. 1255–1295.
O. Brandt and C. Günther. In vitro and in vivo metabolism of testosterone in human and hairless vs. nude mouse skin. Exp. Toxic. Pathol. 48:334–335 (1996).
S. Ahmed, T. Imai, and M. Otagiri. Evaluation of stereoselective transdermal transport and concurrent hydrolisis of several ester prodrugs of propranolol: mechanism of stereoselective permeation. Pharmaceut. Res. 13:1524–1529 (1996).
R. C. O'Neil and J. E. Carless. Influence of side chain on the hydrolysis of hydrocortisone esters. J. Pharm. Pharmacol. 82:10 (1980).
E. Eckle, G.-A. Hoyer, H. Hofmeister, H. Laurent, D. Schomburg, and R. Wiechert. Die Kristall-und Molekülstruktur von Gestoden. Liebigs Ann. Chem. 1988:199–202.
C log P software, Schering AG, 1992.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lipp, R., Laurent, H., Günther, C. et al. Prodrugs of Gestodene for Matrix-Type Transdermal Drug Delivery Systems. Pharm Res 15, 1419–1424 (1998). https://doi.org/10.1023/A:1011957822961
Issue Date:
DOI: https://doi.org/10.1023/A:1011957822961